A kind of preparation method of 1,3-dimethylcyanoacetylurea
A technology of dimethyl cyanoacetyl urea and dimethyl urea, which is applied in 1 field, can solve problems such as the quality of cyanoacetyl urea, the influence of condensation yield, the decomposition of cyanoacetic acid, and the high level of cyanoacetic acid, so as to save acetic anhydride, eliminate damage, and raw materials The effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention provides a kind of preparation method of 1,3-dimethyl cyanacetamide, the method comprises:
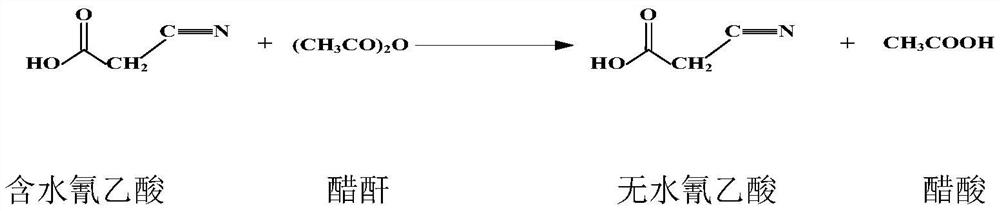
[0028] Step 1: carry out vacuum distillation of cyanoacetic acid under heating and negative pressure, and distill out a large amount of water in cyanoacetic acid. More preferably, it is 0.095-0.098MPa;
[0029] Step 2: after the vacuum rectification is completed, add anhydride-containing acetic acid to the cyanoacetic acid after the rectification, and the anhydride-containing acetic acid is obtained by dissolving acetic anhydride in acetic acid, and the content of the acetic anhydride is 3-5 %, utilize the acetic anhydride of content 3~5% in the anhydride-containing acetic acid and the remaining small amount of water to all react to produce acetic acid, then continue to heat negative pressure distillation, carry out acid with water, obtain anhydrous cyanoacetic acid and acetic acid; The temperature is preferably ≤92°C, more preferably 85-90°C, and the vacu...
Embodiment 1
[0037] With 255kg of cyanoacetic acid, heating, negative pressure carry out vacuum distillation, a large amount of water in the cyanoacetic acid is distilled out, the distillation temperature is 85 ℃, and the vacuum degree is 0.095MPa;
[0038] After the vacuum rectification is completed, add 130 L of anhydride-containing acetic acid, utilize 3% acetic anhydride in the anhydride-containing acetic acid and the remaining small amount of water to all react to produce acetic acid, then continue to heat negative pressure distillation, carry out acid with water, and the temperature of the water with water is 85℃, the vacuum degree is 0.095MPa, and 230kg of anhydrous cyanoacetic acid and 110L of acetic acid are obtained;
[0039] After the water-carrying end point, cool down to 38 ℃ and add 196kg of dimethylurea, complete, then adjust the temperature to be 58 ℃ and add 255kg of acetic anhydride to carry out the condensation reaction, and the condensation reaction temperature reaches 9...
Embodiment 2
[0042] 255kg of cyanoacetic acid was heated and subjected to vacuum distillation under negative pressure, and a large amount of water in the cyanoacetic acid was distilled out, and the distillation temperature was 90°C, and the vacuum degree was 0.098MPa;
[0043] After the vacuum rectification is completed, add 150 L of anhydride-containing acetic acid, utilize 5% acetic anhydride in the anhydride-containing acetic acid and a small amount of water to react to produce acetic acid, then continue to heat negative pressure distillation, carry out acid with water, and the temperature of the water is 90℃, the vacuum degree is 0.098MPa, and 235kg of anhydrous cyanoacetic acid and 130L of acetic acid are obtained;
[0044] After the water-carrying end point, cool down to 42 ℃ and add 197kg of dimethylurea, complete, then adjust the temperature to be 62 ℃ and add 256kg of acetic anhydride to carry out the condensation reaction, and the condensation reaction temperature reaches 98 ℃ to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com