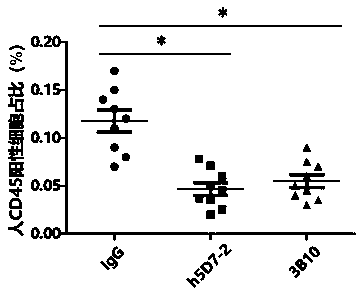
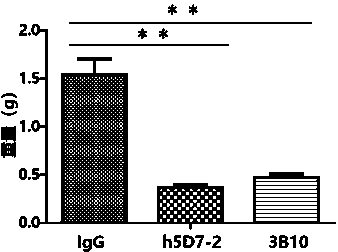
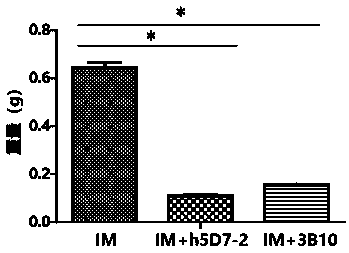
Application of anti-PTN antibody to inhibition of leukemia stem cells and treatment of chronic granulocytic leukemia
An antibody and application technology, applied in the direction of antibody medical components, antibodies, anti-growth factor immunoglobulin, etc., can solve the problems that have not been used to treat chronic myeloid leukemia and the binding ability is not very ideal, and achieve the goal of inhibiting the growth of CML cells Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Preparation of PTN antigen
[0028]The PTN cDNA was inserted into the pET-43.1b(+) vector to construct the PTN prokaryotic expression plasmid, which was designated as pET-43.1b(+)-PTN. Transform the above plasmids into Escherichia coli competent BL21 (DE3), then add the competent cells to 2ml LB containing ampicillin resistance for overnight culture, and inoculate 1% inoculum in 20 mL LB containing ampicillin resistance the next day Culture medium was shaken at 37 °C for 3 h, then IPTG with a final concentration of 0.5 mmol / L was added to induce culture at 30 °C for 6 h. Collect the bacteria by centrifugation, add the lysate and perform ultrasonic crushing, collect the supernatant by centrifugation, and then further purify it through a nickel column to obtain the soluble recombinant antigen protein, digest it with enterokinase to remove the tagged protein carried on the plasmid, and finally obtain the PTN recombinant protein . Because the PTN protein gene i...
Embodiment 2
[0029] Example 2. Preparation of anti-PTN antibody
[0030] The PTN-KLH antigen prepared in Example 1 was injected intraperitoneally into female Balb / c mice together with Freund's complete adjuvant that caused an immune response, for initial immunization, and then injected into the above-mentioned females with the same amount of antigen every 2 weeks. Balb / c mice were immunized intraperitoneally. Finally, the last immunization was carried out 3 days before the fusion with myeloma cells. PTN-KLH antigen was mixed and emulsified with immune adjuvant, and injected intraperitoneally for the last immunization. The immunized Balb / c mouse splenocytes were taken and fused with the myeloma Sp2 / 0 cell line, and the fused cells were treated with Iscove medium (0.1 mM hypoxanthine, 0.4 μM aminopterin) containing 10% serum and 16 μM thymidine), diluted to an appropriate concentration, added to a 96-well plate for cultivation. After 10 days, the cell supernatant was taken, and the primary...
Embodiment 3
[0035] Example 3. Determination of the binding ability of anti-PTN antibodies to PTN
[0036] The antibody plasmid obtained in Example 2 was transfected into CHO-K1 cells to screen for high-expression clones, and cultured in serum-free medium for expansion. Protein A affinity column was used to separate and purify the humanized antibody h5D7-2. The purified antibody was dialyzed with PBS to obtain the PTN humanized antibody h5D7-2 with high purity. The anti-PTN antibody 3B10 will be obtained through the US20040234519 patent, (for its light and heavy chain sequences, please refer to the appendix of the patent specification figure 1 B, the sequence of 1C) and the antibody 3B10 was obtained by the above method as a control antibody. The binding affinities of the humanized anti-PTN antibody h5D7-2 obtained above and the control antibody 3B10 to the PTN protein at 50% effective concentration (EC50) were evaluated by ELISA experiments. The recombinant human PTN protein was diluted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com