Preparation method of anti-bone tumor composite material stent
A composite material and bone tumor technology, applied in the field of the preparation of composite material scaffolds, can solve the problems of local inflammatory response, poor mechanical strength, weak cell adhesion, etc., achieve high impact strength and hardness, reduce burden, and avoid sudden release Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (MTX mass ratio is 1%, MTX-PLA / HA composite bone repair scaffold)
[0033] Preparation of hydroxyapatite by co-precipitation method: 185.4g of calcium chloride and 380g of sodium phosphate dodecahydrate were dissolved in 1L of water respectively. Under the condition of 90℃ water bath and stirring, add the sodium phosphate solution to the calcium chloride solution, adjust the pH to 9~11 with sodium hydroxide solution and hydrochloric acid, and keep stirring for 4 hours. Stand still and age for 12 hours. After aging, wash to remove the by-product sodium chloride, and dry to obtain nano-sized hydroxyapatite.
[0034] Methotrexate powder, hydroxyapatite powder and PLA powder were sieved through 200 meshes respectively. The weight content of methotrexate (MTX) in the mixed raw material is 1%, the weight content of nanoscale hydroxyapatite powder in the mixed raw material is 10%, and the balance is PLA powder. The mixture was added to an internal mixer and mixed for 10 min...
Embodiment 2
[0037] (MTX mass ratio is 0.1%, MTX-PLA / HA composite bone repair scaffold)
[0038] The difference from Example 1 is that the weight content of methotrexate (MTX) in the mixed raw material is 0.1%, and the weight content of nanoscale hydroxyapatite powder in the mixed raw material is 9%.
Embodiment 3
[0040](MTX mass ratio is 5%, MTX-PLA / HA composite bone repair scaffold)
[0041] The difference from Example 1 is that the weight content of methotrexate (MTX) in the mixed raw material is 5%, and the weight content of nanoscale hydroxyapatite powder in the mixed raw material is 11%.
[0042] In the above examples, the specific dosage of methotrexate needs to be regulated according to the patient's own condition and doctor's order.
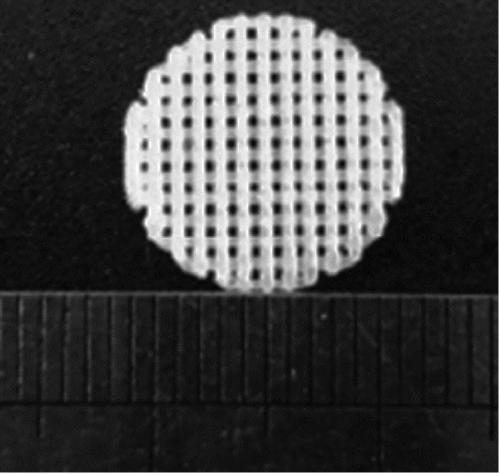
[0043] figure 1 It is a sample picture of the MTX-PLA / HA composite anti-tumor scaffold. It can be seen that the voids in the anti-tumor scaffold have a pore size of about 300 microns. The existence of porous voids accelerates the degradation of polylactic acid in the human body and reduces the drug release rate. difficulty. And the existence of pores provides convenience for the later generation of new bone. The stent sample is not limited to the disc shape, and the specific shape can be adjusted by itself according to the CT data.
[0044] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap