A class of 2-substituted tetrahydroquinoline compounds and their derivatives, preparation methods and applications
A tetrahydroquinoline, compound technology, applied in the directions of organic chemistry, drug combination, antitumor drugs, etc., can solve problems such as damage to sustainability, and achieve the effect of green preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
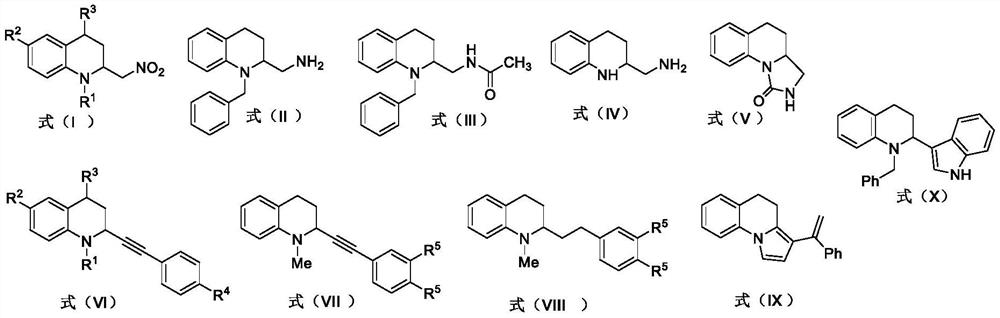
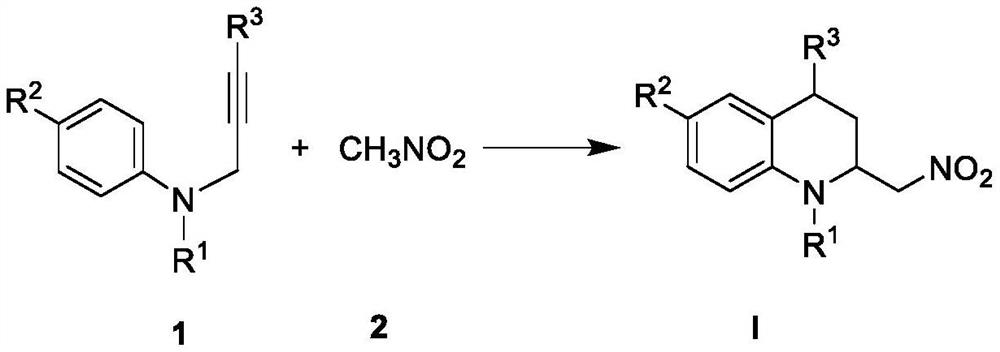
[0080] Preparation of compound I-1 (N-benzyl-2-nitromethyl-1,2,3,4-tetrahydroquinoline)
[0081] R 1 is benzyl; R 2 is hydrogen; R 3 for hydrogen
[0082] Take compound 1-1 (0.25mmol) in a 15ml thick-walled pressure bottle, weigh the metal catalyst ZnBr 2 (0.025mmol, 5.7mg) was added to a thick-walled pressure bottle, and nitromethane (500 μl) was added to the pressure bottle, N 2 Protection, stirring reaction at 100 ° C for 24h. After the reaction, the reaction solution was transferred to a 25ml eggplant-shaped bottle using dichloromethane, concentrated under reduced pressure, and the crude product was subjected to silica gel column chromatography, and the eluent was petroleum ether:ethyl acetate=30:1 (v / v) , separation and purification to obtain pure product, light yellow solid, yield: 65% (45.8mg); 1 H-NMR (400MHz, CDCl 3 ): δ7.30(t, J=7.3Hz, 2H), 7.23(dd, J=14.1, 7.1Hz, 3H), 7.04(d, J=7.4Hz, 1H), 6.99(t, J=7.8Hz ,1H),6.67(t,J=7.3Hz,1H), 6.55(d,J=8.3Hz,1H),4.62(ABq,...
Embodiment 2
[0084] Preparation of compound I-2 (N-phenyl-2-nitromethyl-1,2,3,4-tetrahydroquinoline)
[0085] R 1 is phenyl; R 2 is hydrogen; R 3 for hydrogen
[0086] Get compound 1-2 (0.25mmol) in 15ml thick-walled pressure bottle, weigh metal catalyst ZnBr 2 (0.025mmol, 5.7 mg) into a thick-walled pressure bottle, measure nitromethane (500μl) into the pressure bottle, N 2 Protection, stirring reaction at 100 ° C for 24h. After the reaction, the reaction solution was transferred to a 25ml eggplant-shaped bottle using dichloromethane, concentrated under reduced pressure, and the crude product was subjected to silica gel column chromatography, and the eluent was petroleum ether:ethyl acetate=30:1 (v / v) , separation and purification to obtain pure product, yellow oily liquid; yield: 61% (40.8mg); 1 H-NMR (400MHz, CDCl 3 ): δ7.36(t, J=7.8Hz, 2H), 7.20-7.14(m, 3H), 7.09(d, J=7.5Hz, 1H), 6.96(t, J=7.7Hz, 1H), 6.79 (t, J=7.4Hz, 1H), 6.62(d, J=8.2Hz, 1H), 4.63-4.44(m, 3H), 2.93-2.84(m, 2...
Embodiment 3
[0088] Preparation of compound I-3 (N-allyl-2-nitromethyl-1,2,3,4-tetrahydroquinoline)
[0089] R 1 is allyl; R 2 is hydrogen; R 3 for hydrogen
[0090] Take compound 1-3 (0.25mmol) in a 15ml thick-walled pressure bottle, weigh the metal catalyst ZnBr 2 (0.025mmol, 5.7 mg) into a thick-walled pressure bottle, measure nitromethane (500μl) into the pressure bottle, N 2 Protection, stirring reaction at 100 ° C for 24h. After the reaction, the reaction solution was transferred to a 25ml eggplant-shaped bottle using dichloromethane, concentrated under reduced pressure, and the crude product was subjected to silica gel column chromatography, and the eluent was petroleum ether:ethyl acetate=30:1 (v / v) , separation and purification to obtain pure product, yellow oily liquid, yield: 55% (31.9mg); 1 H-NMR (400MHz, CDCl 3 ): δ7.08(t, J=7.8Hz, 1H), 7.03(d, J=7.3Hz, 1H), 6.69(t, J=7.3Hz, 1H), 6.63(d, J=8.3Hz, 1H ),5.85(ddt,J=15.6,10.0,4.9Hz,1H), 5.24-5.16(m,2H),4.53(dd,J=11.5,5.6Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com