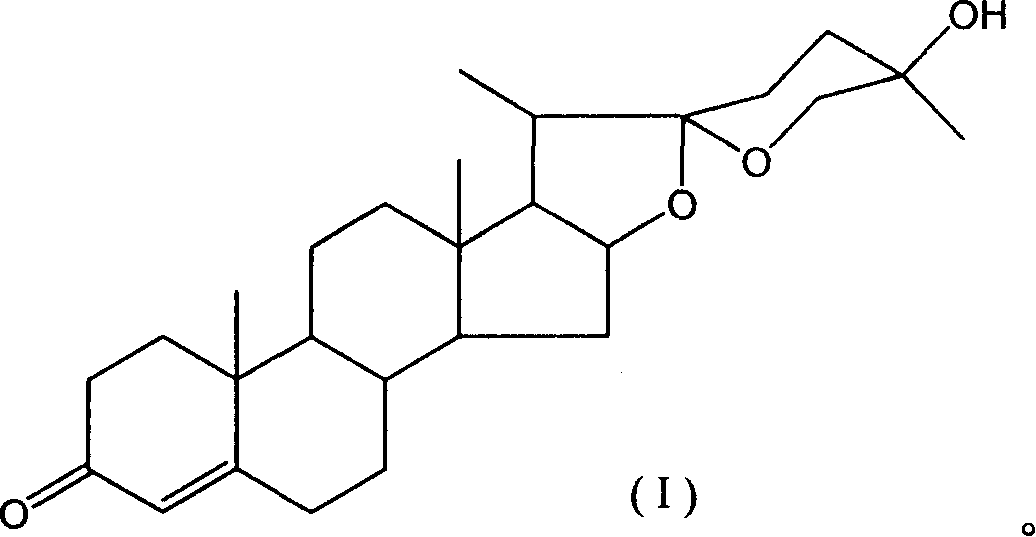
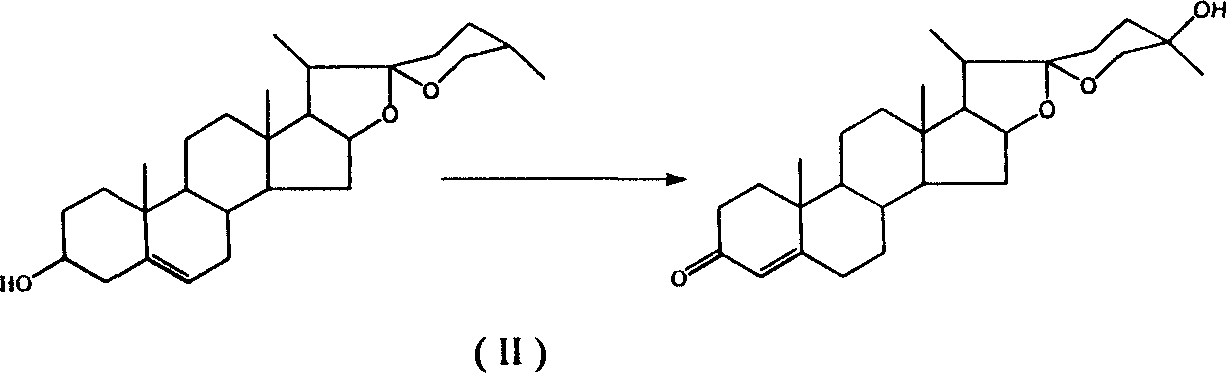
25-hydroxy dioscin ketone, and its preparing method and use
A technology of diosgenone and hydroxyl diosgen, which is applied in the application field of 25-hydroxydioscinone, can solve problems such as poor selectivity, and achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of 25-hydroxydiosgenone:
[0028] The microorganism used: Streptomyces virginiae IBL-14, the preservation number is CCTCC M206045.
[0029] Streptomyces virginiana was cultured at 29°C to the end logarithmic phase of growth, and diosgenin dissolved in hot ethanol was added at a concentration of 0.4 g / ml to a final concentration of 0.0015 mol / L, transformed for 48 hours, and the reaction was stopped. The volume of ethyl acetate was extracted three times, the extract was concentrated, concentrated under reduced pressure, passed through a silica gel column, and 0.0050 mol of 25-hydroxydiosgenone could be obtained per liter of reaction solution.
[0030] The components and content of the culture medium are as follows:
[0031] Ammonium chloride 3 grams per liter; dipotassium hydrogen phosphate 1.55 grams per liter; sodium dihydrogen phosphate 0.85 grams per liter; glucose 3 grams per liter; yeast powder 3 grams per liter; corn steep liquor 3 grams per liter; cy...
Embodiment 2
[0036] Preparation of 25-hydroxydiosgenone:
[0037] Microorganism used: Streptomyces virginiae, selected from the Culture Collection.
[0038] Streptomyces virginiana was cultured at 29°C to the end logarithmic phase of growth, and diosgenin dissolved in hot ethanol was added at a concentration of 0.4 g / ml to a final concentration of 0.0015 mol / L, transformed for 48 hours, and the reaction was stopped. The volume of ethyl acetate was extracted three times, the extract was concentrated, concentrated under reduced pressure, passed through a silica gel column, and 0.0040 mol of 25-hydroxydiosgenone could be obtained per liter of reaction solution.
[0039] The components and contents of the culture medium are the same as in Example 1.
Embodiment 3
[0041] Preparation of 25-hydroxydiosgenone:
[0042] Microorganisms used: Streptomyces lavendulae, selected from the Culture Collection.
[0043] Streptomyces lavender was cultured at 28°C to the end of logarithmic growth, and diosgenin dissolved in hot ethanol was added at a concentration of 0.4 g / ml to a final concentration of 0.0015 mol / L, transformed for 48 hours, and the reaction was stopped with an equal volume of Ethyl acetate was extracted three times, the extract was concentrated, concentrated under reduced pressure, passed through a silica gel column, and 0.0040 mol of 25-hydroxydiosgenone could be obtained per liter of reaction solution.
[0044] Component and content of culture medium are the same as example 1:
[0045] Identification of the obtained product: colorless needle-like crystals; mp234-236°C; MS (EI) m / z (%): 428 (0.19) (M+, C27H40O4), 397 (66.90), 343 (3.43), 340 (94.49) , 298 (13.63), 283 (2.11), 269 (100.00), 155 (73.85).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com