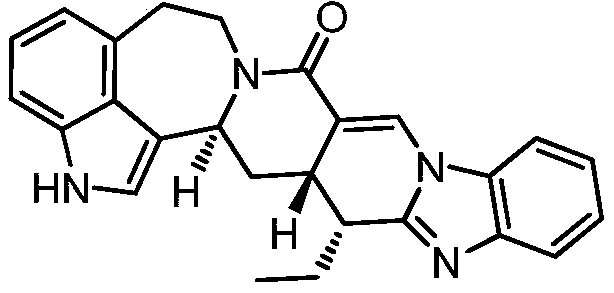
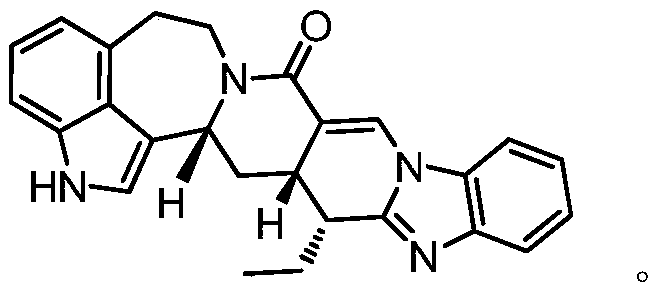
Azepineindole alkaloid and preparation and anti-malarial application thereof
An azepine and indole technology, which is applied in the preparation of aza-indole alkaloids and enzymatic chemical methods, and the application field in the preparation of anti-malarial drugs, can solve the problem of the stereoselective PS method and the synthetic method without manual Sexual selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
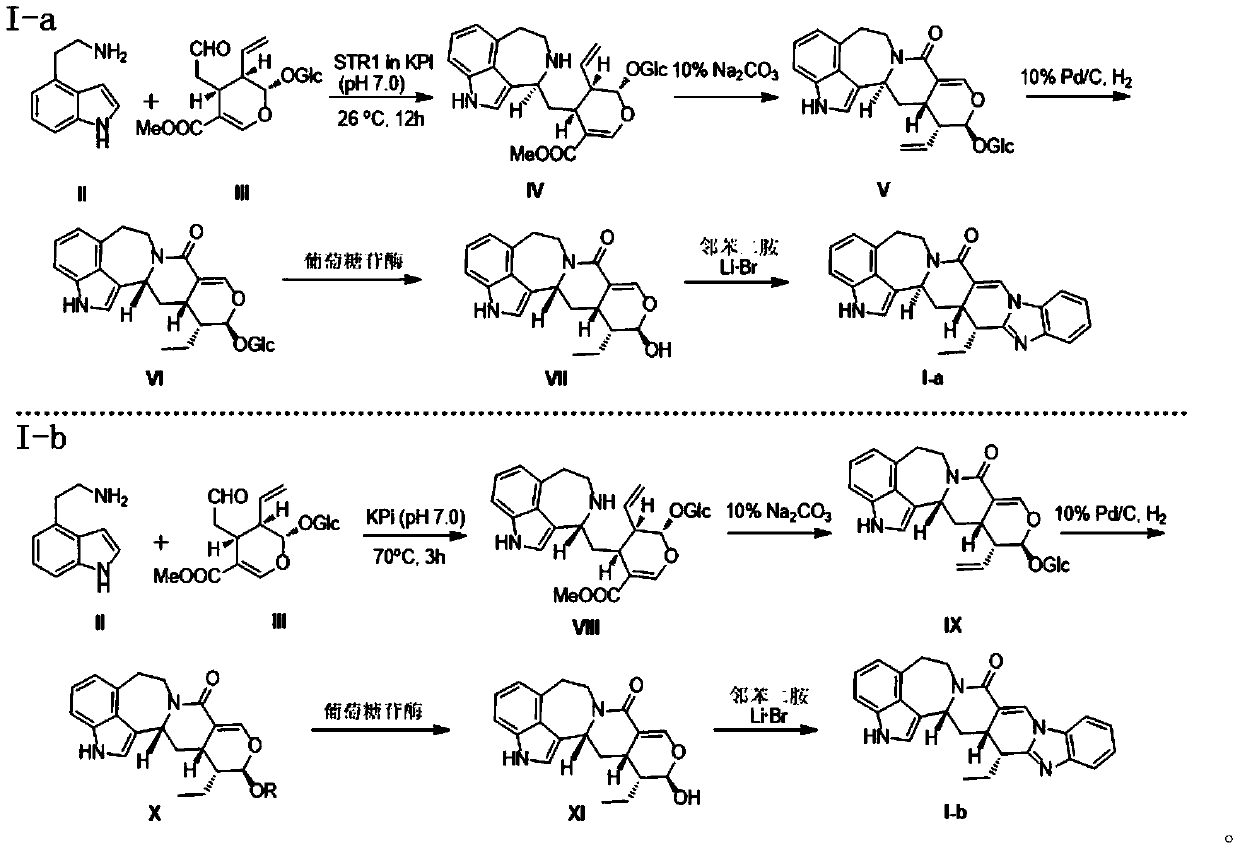
[0019] Embodiment 1: Preparation of azazoindole isososidine (IV)
[0020] Dissolve compound II (0.4g, 2.5mmol) and compound III (1.1g, 2.5mmol) in KPi (50mM, pH=7.0, 200ml) respectively, add STR1 5mg, and incubate at 26°C for 12h. After the reaction is complete, no After purification, it was directly used in the reaction of Example 2.
Embodiment 2
[0021] Example 2: (10S, 11R, 11aS, 12aR)-10-(((2S, 3R, 4S, 5S, 6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H -pyran-2-yl)oxy)-11-ethenyl 10,11,11a,12,12a,14 hexahydro-4H-pyrano[4",3":4',5']pyrido Preparation of [1',2':1,2]aza[3,4,5-cd]indol-7(5H)-one (V).
[0022] Add 5% sodium carbonate solution (500ml) to the reaction solution of Example 1 and mix to obtain a suspension, and react at 70°C for 2 hours. After the reaction is completed, the solvent is removed by distillation under reduced pressure, and flash column chromatography obtains a yellow solid 4 , 14g, yield 86%. 1 HNMR (500MHz, CD 3 OD): δ7.35 (1H, d, J = 2.0Hz), 7.21 (1H, d, J = 8.0Hz), 7.18 (1H, d, J = 1.5Hz), 7.03 (1H, t, J = 8.0 Hz),6.84(1H,d,J=8.0Hz),5.64(1H,dt,J=17.0,10.0Hz),5.39(1H,d,J=2.0Hz),5.36(1H,dd,J=17.0 ,2.0Hz),5.29(1H,dd,J=10.0,2.0Hz),4.98(1H,d,J=5.0Hz),4.76(1H,dt,J=13.5,3.5Hz),4.58(1H,d ,J=8.0Hz),3.86(1H,dd,J=12.0,2.0Hz),3.64(1H,dd,J=12.0,6.0Hz),3.49(1H,m),3.07-3.26(6H,m) ,2.97(1H,ddd,J=8.5...
Embodiment 3
[0023] Example 3: Preparation of reduced azaindole lactamized isosidine (VI)
[0024] Compound V (3.00g, 6.00mmol) was dissolved in 50ml of methanol, and 10% palladium carbon (300mg) was added to react overnight at room temperature. The palladium carbon was removed by filtration, and the solvent was distilled off under reduced pressure to obtain 2.98g of a yellow solid with a yield of 98%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com