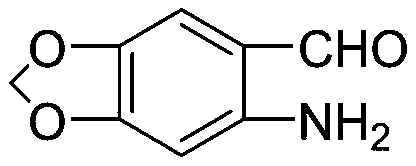
Preparation method of medical intermediate 6-amino heliotropin
A technology of amino piperonal and intermediates, applied in directions such as organic chemistry, can solve the problems of consuming raw material cost and time cost, the yield of 6-nitro piperonal is only about 50%, difficult to separate, etc., saving time and cost and material costs, saving time and labor costs, and the effect of broad industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of pharmaceutical intermediate 6-aminopiperonal, comprising the following steps:
[0038] S1. Under ice bath, add 100g of piperonal into 500ml of concentrated nitric acid to react for 0.5h, then pour into 10L of water;
[0039] S2. The above-mentioned product is extracted with 200ml of dichloromethane, and the organic phase is collected;
[0040] S3 Add potassium permanganate with 2 times the molar amount of piperonal to the organic phase, and react for 3 hours at 90° C. with mechanical stirring at a rotational speed of 200 rpm. Then filter to remove the solid mixture of potassium permanganate and manganese dioxide, collect the filtrate;
[0041] S4. Add the palladium carbon catalyst that consumption is 0.2wt% of piperonal to the filtrate, and under the mixed gas of hydrogen and argon gas (1:1, n / n), react at 80 ℃ to obtain 6-aminopiperone aldehyde crude product, and the crude product was recrystallized to obtain 6-aminopiperonal with a yield of 4...
Embodiment 2
[0049] A preparation method of pharmaceutical intermediate 6-aminopiperonal, comprising the following steps:
[0050] S1. Under ice bath, add 100g piperonal into 500ml concentrated nitric acid to react for 2h, then pour into 10L water;
[0051] S2. The above product is extracted with 200ml of ethyl acetate, and the organic phase is collected;
[0052]S3 Add potassium permanganate with 4 times the molar amount of piperonal to the organic phase, and react for 3 hours at 120° C. under the condition of mechanical stirring, the rotating speed of mechanical stirring is 500 rpm. Then filter to remove the solid mixture of potassium permanganate and manganese dioxide, collect the filtrate;
[0053] S4. Add the palladium carbon catalyst that consumption is 0.5wt% of piperonal to the filtrate, and under the mixed gas of hydrogen and argon gas (4:1, n / n), react at 100 ℃ to obtain 6-aminopiperone aldehyde crude product, and the crude product was recrystallized to obtain 6-aminopiperonal ...
Embodiment 3
[0061] A preparation method of pharmaceutical intermediate 6-aminopiperonal, comprising the following steps:
[0062] S1. Under ice bath, add 100g piperonal into 500ml concentrated nitric acid to react for 2h, then pour into 10L water;
[0063] S2. The above product is extracted with 200ml of ethyl acetate, and the organic phase is collected;
[0064] S3 Add potassium permanganate with 3 times the molar amount of piperonal to the organic phase, and react for 3 hours at 100° C. with mechanical stirring at 300 rpm. Then filter to remove the solid mixture of potassium permanganate and manganese dioxide, collect the filtrate;
[0065] S4. Add the palladium carbon catalyst that consumption is 0.4wt% of piperonal to the filtrate, and under the mixed gas of hydrogen and argon gas (2:1, n / n), react at 90 ℃ to obtain 6-aminopiperone aldehyde crude product, and the crude product was recrystallized to obtain 6-aminopiperonal with a yield of 46%.
[0066] Wherein, the solvent used for ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap