A kind of gas-solid phase joint production method of styrene and benzaldehyde
A technology of joint production and styrene, applied in chemical instruments and methods, preparation of organic compounds, hydrocarbons, etc., can solve the problems of high energy consumption, benzaldehyde products containing halogens, etc., and achieve low operating costs and easy continuity Production, to achieve the effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
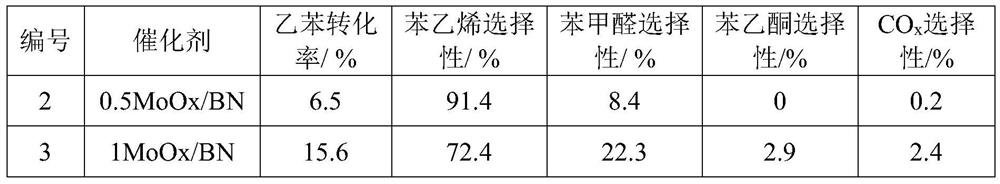
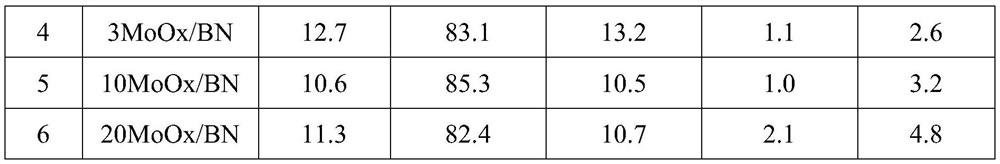
[0053] The preparation process is the same as that of Comparative Example 2, but the carrier is the modified boron nitride obtained by S4 in Comparative Example 1, the catalyst synthesized by different metal salt types and the concentration of different metal salt solutions, and its number 2~10 and preparation conditions The corresponding relationship is shown in Table 1.
Embodiment 2
[0055] The preparation process is the same as in Comparative Example 2, but the carrier is commercial boron nitride (BN-P), the concentration of ammonium molybdate aqueous solution is 0.035g / mL, and the obtained MoOx / BN-P catalyst corresponds to No. 11 in Table 1.
[0056] Table 1 Correspondence between different catalysts and preparation conditions
[0057] Numbering catalyst Metal / wt% metal salt solvent Concentration / g / mL Calcination temperature / ℃ 1 R-BN 0 0 0 0 600 2 0.5MoOx / BN 0.5 Ammonium molybdate water 0.018 600 3 1MoOx / BN 1 Ammonium molybdate water 0.035 600 4 3MoOx / BN 3 Ammonium molybdate water 0.105 600 5 10MoOx / BN 10 Ammonium molybdate water 0.350 600 6 20MoOx / BN 20 Ammonium molybdate water 0.700 600 7 1CrOx / BN 1 Chromium nitrate water 0.140 600 8 1ZrOx / BN 1 Zirconyl nitrate water 0.054 600 9 1VOx / BN 1 Ammonium metavanadate Methanol 0....
Embodiment 3
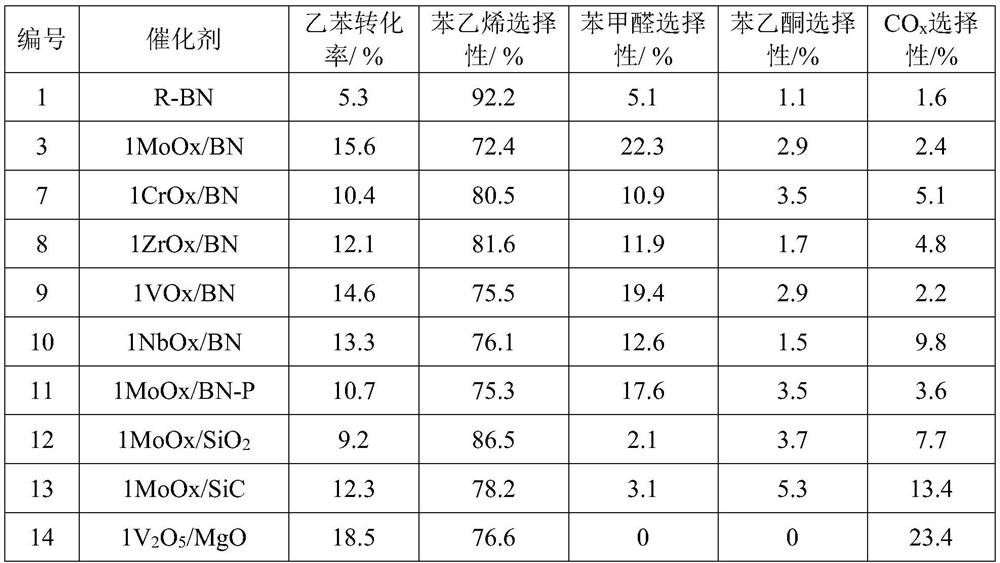
[0059] Effects of Different Catalysts on Ethylbenzene Conversion, Styrene and Benzaldehyde Selectivity
[0060] Ethylbenzene and oxygen were used as raw materials to evaluate the performance of the gas-phase selective oxidation reaction in a fixed-bed reactor. The reaction conditions were as follows: a fixed-bed reactor with an inner diameter of 8 mm was filled with 0.1 g of catalyst, normal pressure, reaction temperature 510 ° C, N 2 / O 2 =9 (mol), ethylbenzene partial pressure is 5.6kPa, ethylbenzene / oxygen=1 (mol); after the reaction is stable, the reaction raw materials and products are detected and analyzed by online chromatography. The corresponding relationship between different catalysts and catalytic activity is shown in Table 2.
[0061] Comparing the catalyst performances of No. 3, 11, 12, and 13 with the same active component, the selected modified boron nitride or commercial boron nitride as the carrier can significantly improve the selectivity of high value-adde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com