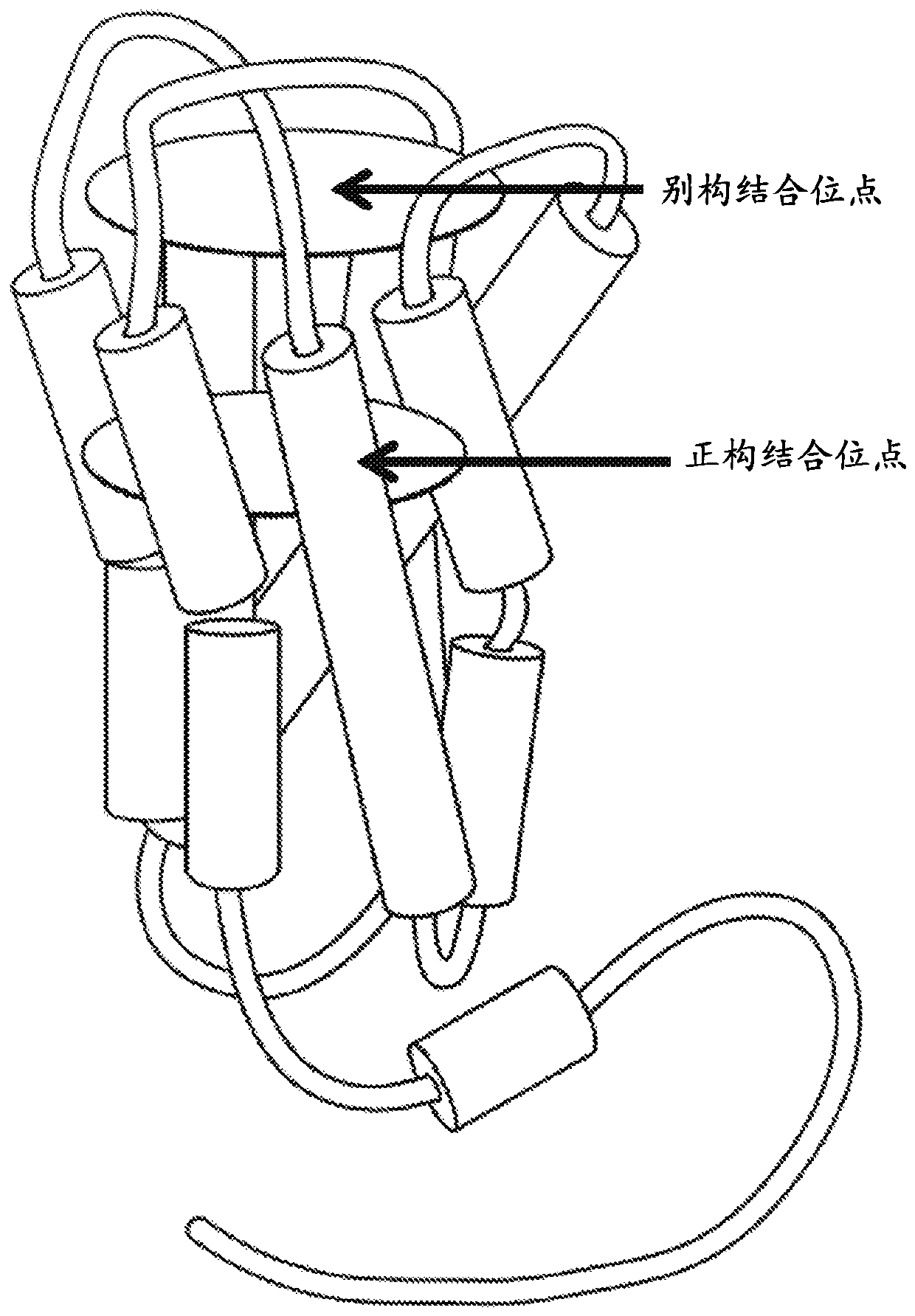
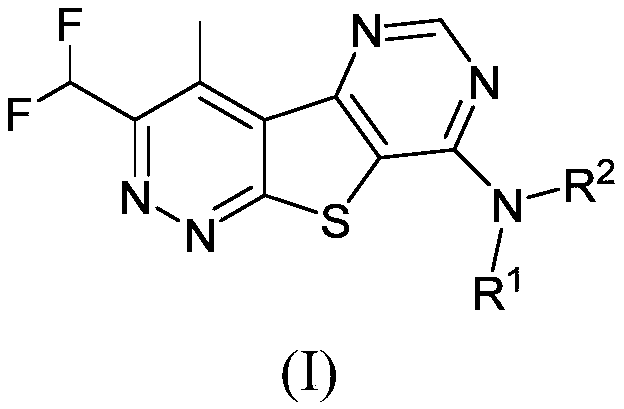
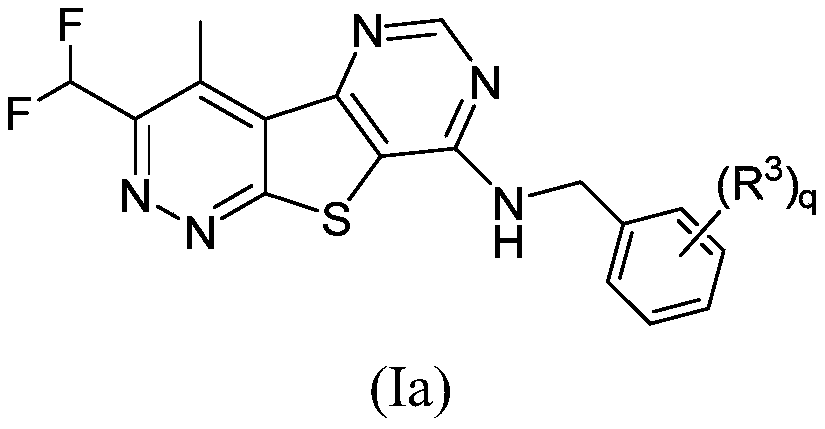
Positive allosteric modulators of the muscarinic acetylcholine receptor m4
A C1-C4, compound technology, applied in the field of positive allosteric modulators of muscarinic acetylcholine receptor M4, can solve problems such as limiting clinical utility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0294] Example 1. General Amine Synthesis
[0295] The following is an exemplary synthesis of one of the amines used to prepare the compounds disclosed herein.
[0296]
[0297] 4-(2-Hydroxypropan-2-yl)benzonitrile. To a solution of 4-iodobenzonitrile (10.0 g, 43.7 mmol, 1.0 equiv) in THF (218 mL) was added n-butyllithium (2.5 M in hexane, 22.7 mL) dropwise at -78 °C. 56.8 mmol, 1.3 eq) to keep the temperature below -70 °C. After 1 hour, acetone (32.0 mL, 436.6 mmol, 10.0 equiv) was added while maintaining the temperature below -70 °C. Remove the dry ice bath. After 16 h at room temperature, add saturated NH 4 Cl solution (100 mL), then EtOAc (250 mL) was added. The layers were separated. The aqueous layer was extracted with EtOAc (2 x 200 mL). The combined organic layers were washed with brine, dried (Na 2 SO 4 ), filtered and concentrated. The residue was purified by flash column chromatography on silica gel (0%-60% EtOAc / hexanes) to afford the title compound (4...
example 2
[0299] Example 2.2-(4-(((3-(Difluoromethyl)-4-methylpyrimido[4',5':4,5]thieno[2,3-c]pyridazin-8-yl )amino)methyl)phenyl)propan-2-ol (2-(4-(((3-(Difluoromethyl)-4-methylpyrimido[4',5':4,5]thieno[2,3-c ]pyridazin-8-yl)amino)methyl)phenyl)propan-2-ol) (compound 1)
[0300]
[0301] Compound reference numbers in the following experiments correspond to those used in Scheme 1 in the Detailed Description section.
[0302] 3-Chloro-5,6-dimethyl-1-oxo-pyridazin-1-ium-4-carbonitrile (ii). To a solution of 3-chloro-5,6-dimethylpyridazine-4-carbonitrile (i) (10.0 g, 59.7 mmol, 1.0 equiv) in DCM (298 mL, 0.2 M) at 0 °C in small amounts 3-Chloroperoxybenzoic acid (40.1 g, 179 mmol, 3.0 equiv) was added in portions. The ice bath was removed, and the mixture was stirred at room temperature. After 16 hours, the reaction mixture was diluted with DCM and washed with NaHCO 3 Wash with saturated solution (2x 200 mL). The organic layer was washed with brine, washed with Na 2 SO 4 Dry, fi...
example 3
[0317] Example 3. Biological Activity
[0318] A. Cell lines expressing muscarinic acetylcholine receptors
[0319] Use Lipofectamine2000 to human M 4 cDNA together with chimeric protein G qi5 Transfected into Chinese Hamster Ovary (CHO-K1) cells purchased from American Type Culture Collection. hM 4 -G qi5 Cells were cultured in Ham's F-12 medium containing 10% heat-inactivated fetal bovine serum (FBS), 20 mM HEPES, 50 μg / mL G418 sulfate, and 500 μg / mL hygromycin B. will rM 4 -G qi5 Cells were cultured in DMEM containing 10% heat-inactivated FBS, 20 mM HEPES, 400 μg / mL G418 sulfate, and 500 μg / mL hygromycin B.
[0320] B. Cell-Based Functional Assays of Muscarinic Acetylcholine Receptor Activity
[0321] For high-throughput measurement of agonist-induced increases in intracellular calcium, stable expression of muscarinic Recipient CHO-K1 cells were plated in growth medium lacking G418 and hygromycin. at 37°C and 5% CO 2 , incubate the cells overnight. The next day...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com