Absorbable Implantable Devices
An implantable and instrumental technology, applied in the direction of prosthesis, surgery, coating, etc., can solve the problems of prolonging the cycle of iron-based stents, hemolysis, and increasing the risk of thrombus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
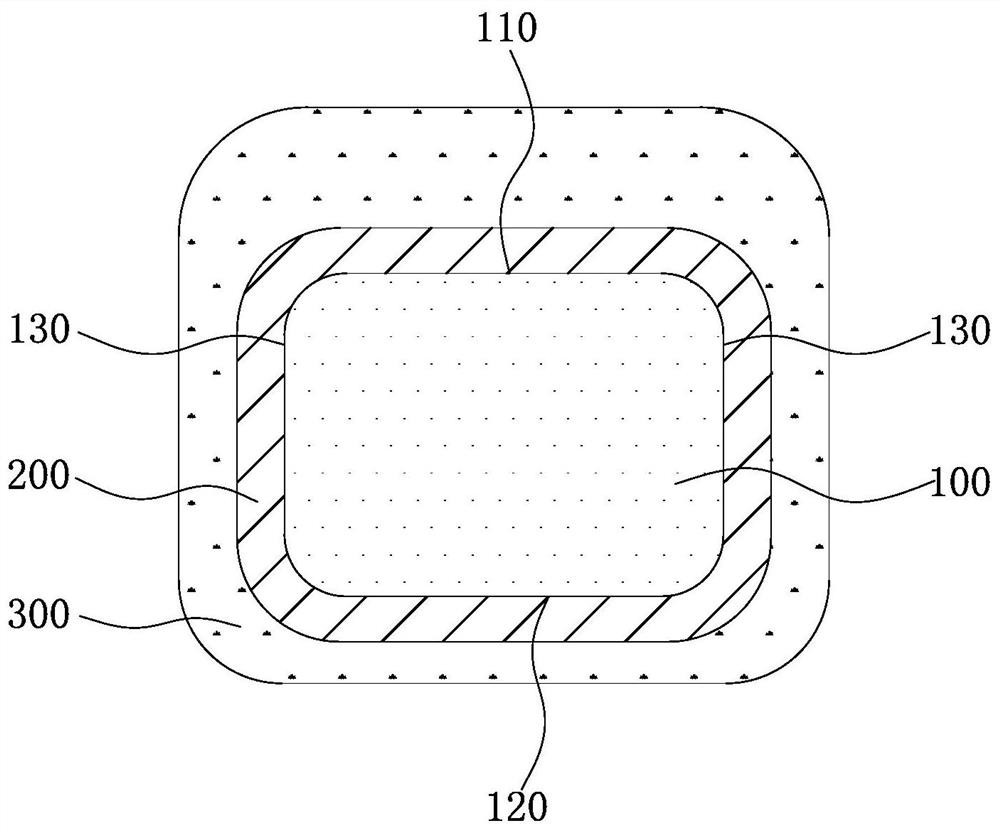
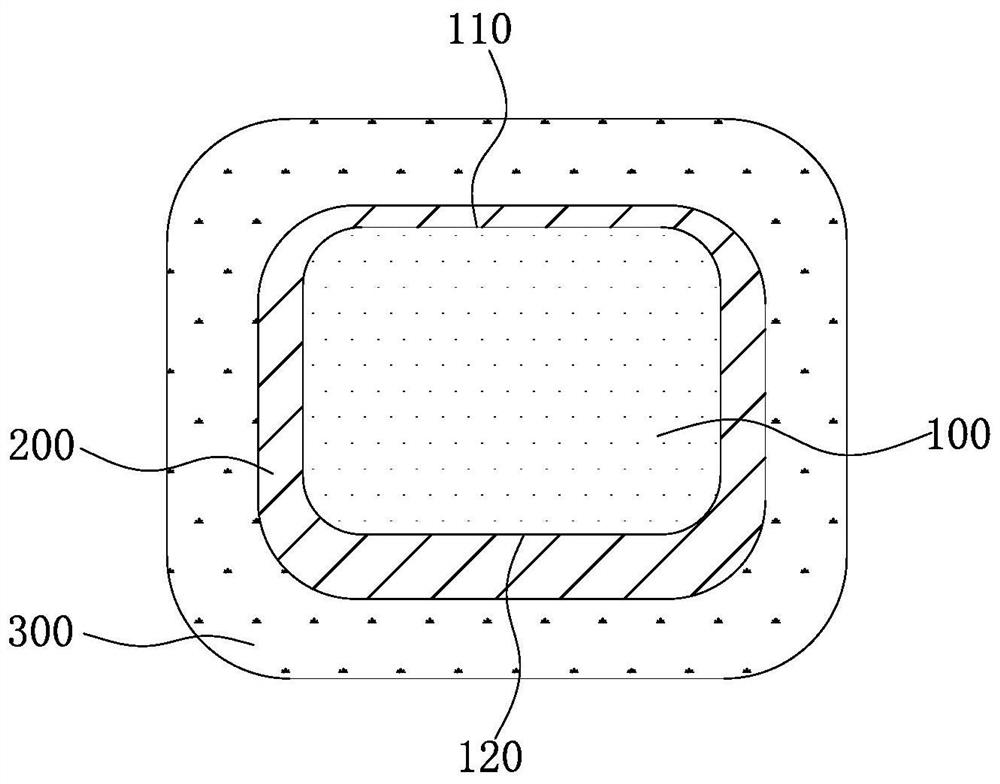
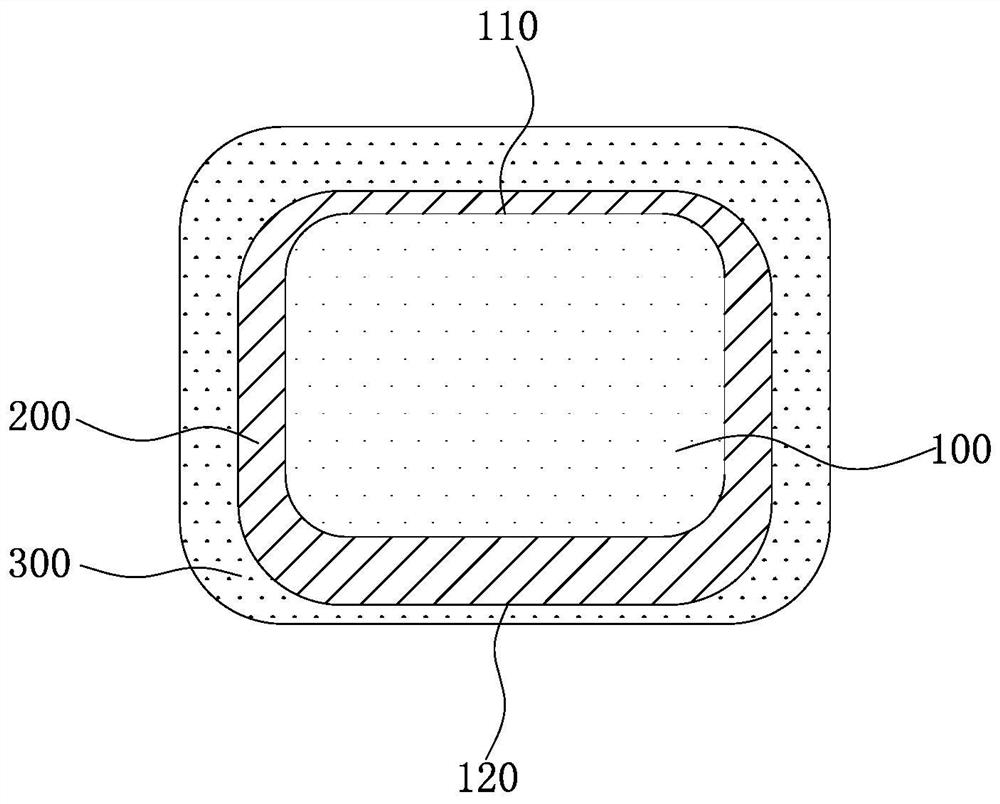
[0074] An absorbable iron-based stent, the preparation method of which is as follows: after laser engraving and polishing by evaporation method, the mass is 3.8mg, the wall thickness is 50μm, the inner diameter is 1.45mm, and the nitrogen content is 0.05wt.% 30008 specification Galvanized on the nitrided iron-based substrate, a zinc-containing protective layer covering the outer wall, inner wall and side wall of the iron-based substrate is formed on the iron-based substrate, and the thickness of the part of the zinc-containing protective layer located on the outer wall and inner wall of the iron-based substrate Both are 0.4 μm. Subsequently, a mixed solution of poly(racemic lactic acid) and ethyl acetate was inkjet printed on the zinc-containing protective layer, wherein the weight-average molecular weight of the poly(racemic lactic acid) was 200,000, and the ethyl acetate was volatilized to completely cover the zinc-containing protective layer. corrosion-promoting layer. The...
Embodiment 2
[0077] An absorbable iron-based stent, the preparation method of which is as follows: after laser engraving and polishing, the electroplating method is used to obtain a 30008-gauge infiltration with a mass of 3.8 mg, a wall thickness of 50 μm, an inner diameter of 1.45 mm, and a nitrogen content of 0.1 wt.%. Galvanized on the nitrogen-iron-based substrate, a zinc-containing protective layer covering the outer wall, inner wall and side wall of the iron-based substrate is formed on the iron-based substrate, and the thicknesses of the parts of the zinc-containing protective layer located on the outer wall and the inner wall of the iron-based substrate are respectively 0.8μm and 1.2μm. Subsequently, a mixed solution of polyracemic lactic acid and ethyl acetate is sprayed on the zinc-containing protective layer, wherein the weight average molecular weight of the polyracemic lactic acid is 200,000. When spraying, a mandrel with a diameter of 0.8mm is added in the stent, and the mand...
Embodiment 3
[0080] An absorbable iron-based stent, the preparation method of which is as follows: after laser engraving and polishing by evaporation method, the mass is 3.8mg, the wall thickness is 50μm, the inner diameter is 1.45mm, and the nitrogen content is 0.06wt.% 30008 specification Galvanized on the nitrided iron-based substrate, a zinc-containing protective layer covering the outer wall, inner wall and side wall of the iron-based substrate is formed on the iron-based substrate, and the thickness of the part of the zinc-containing protective layer located on the outer wall and inner wall of the iron-based substrate 2.5 μm and 2 μm, respectively. Subsequently, a mixed solution of polyracemic lactic acid and ethyl acetate is sprayed on the zinc-containing protective layer, wherein the weight average molecular weight of the polyracemic lactic acid is 200,000. When spraying, a mandrel with a diameter of 0.5mm is added in the stent, and the mandrel can prevent part of the mixed solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap