Patents
Literature
37 results about "Absorbable Implants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Implants constructed of materials designed to be absorbed by the body without producing an immune response. They are usually composed of plastics and are frequently used in orthopedics and orthodontics.
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20060013857A1Lower potentialMinimally inhibit osteogenesis and subsequent bone healingSurgical adhesivesSkeletal disorderBarium saltApatite
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters unsubstituted and N-substituted pyrrolidones of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
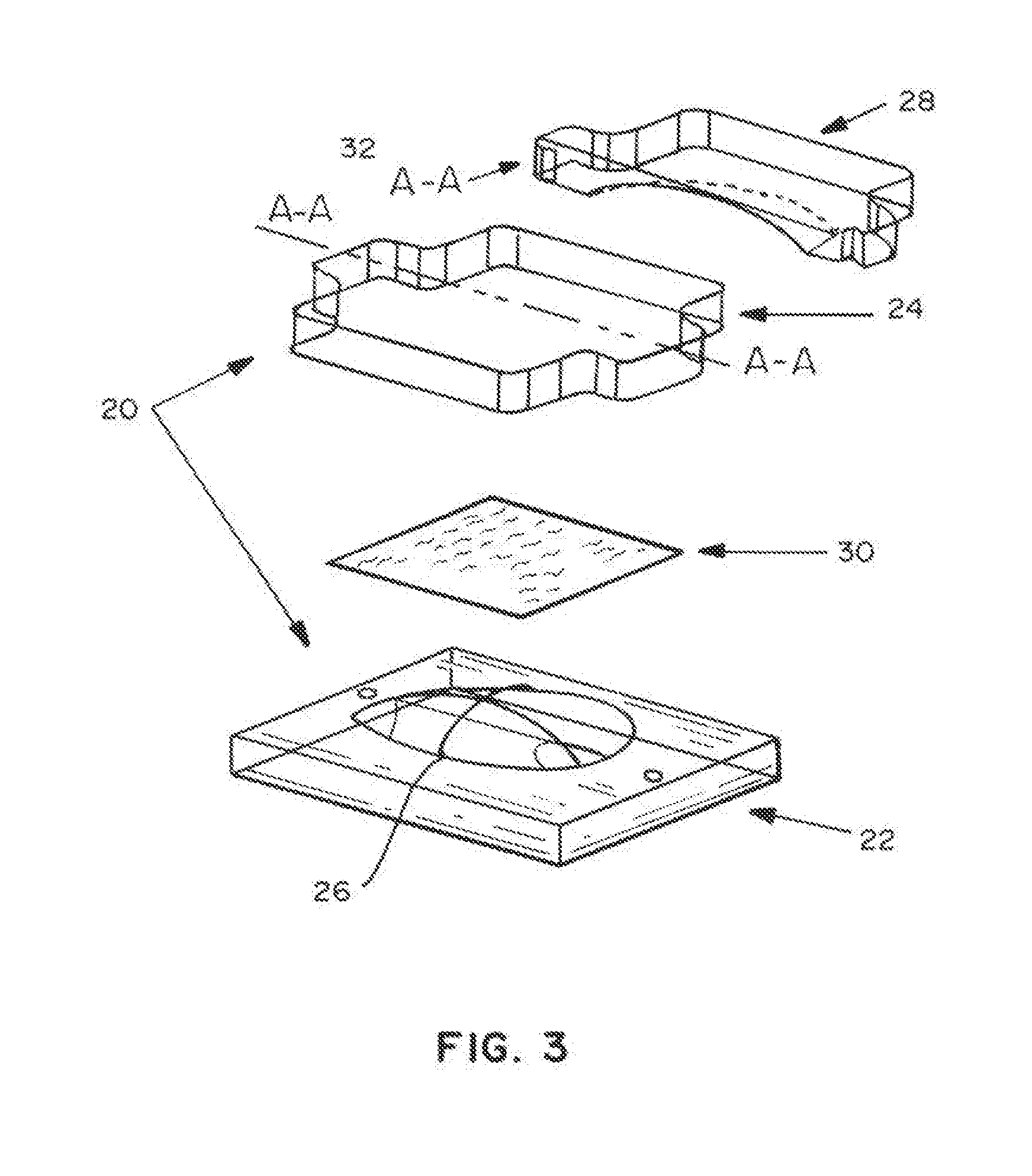
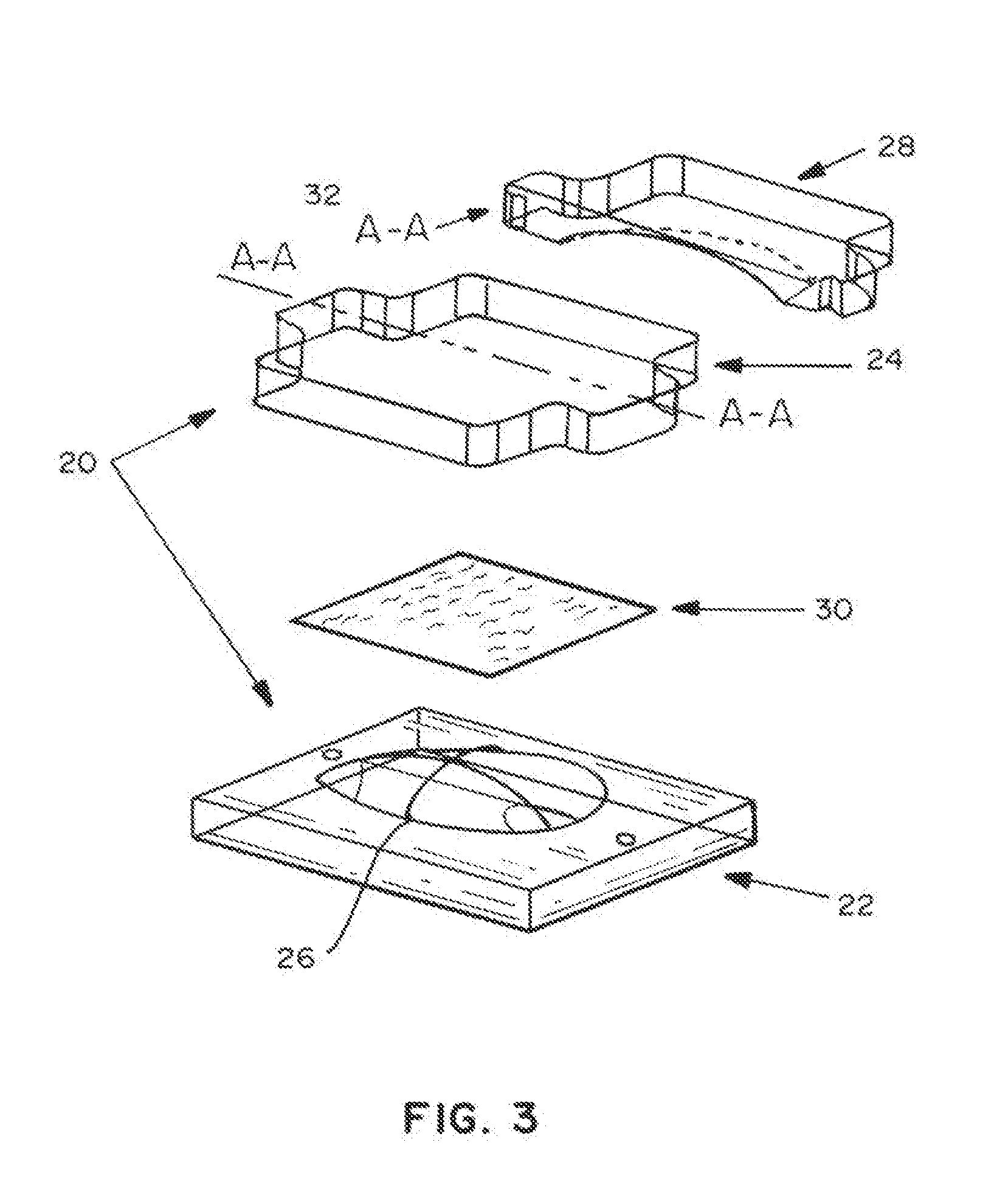
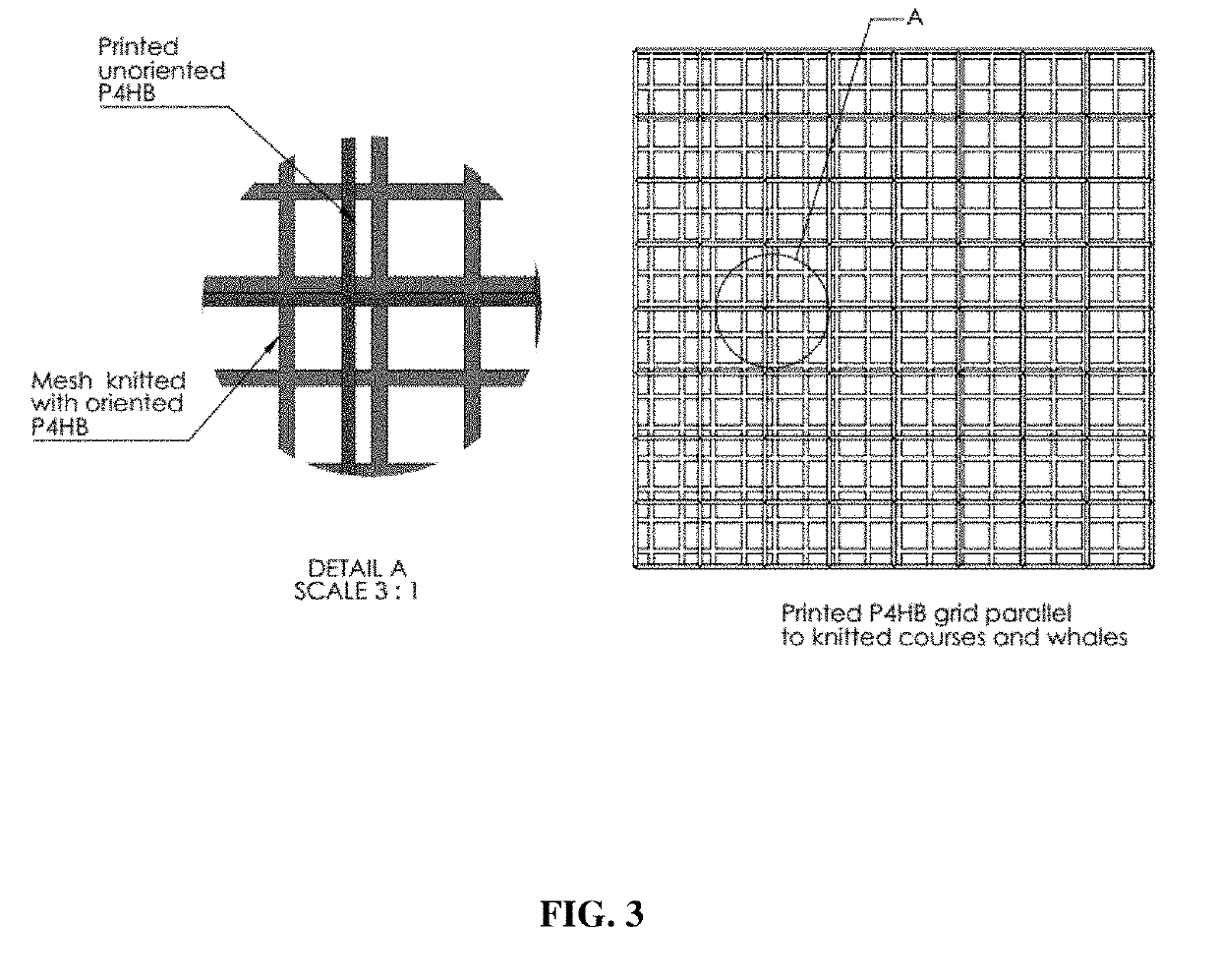
Absorbable implants for plastic surgery
ActiveUS20150223928A1Solve the lack of mechanical propertiesMinimization requirementsMammary implantsDiagnosticsPullout strengthThree dimensional shape
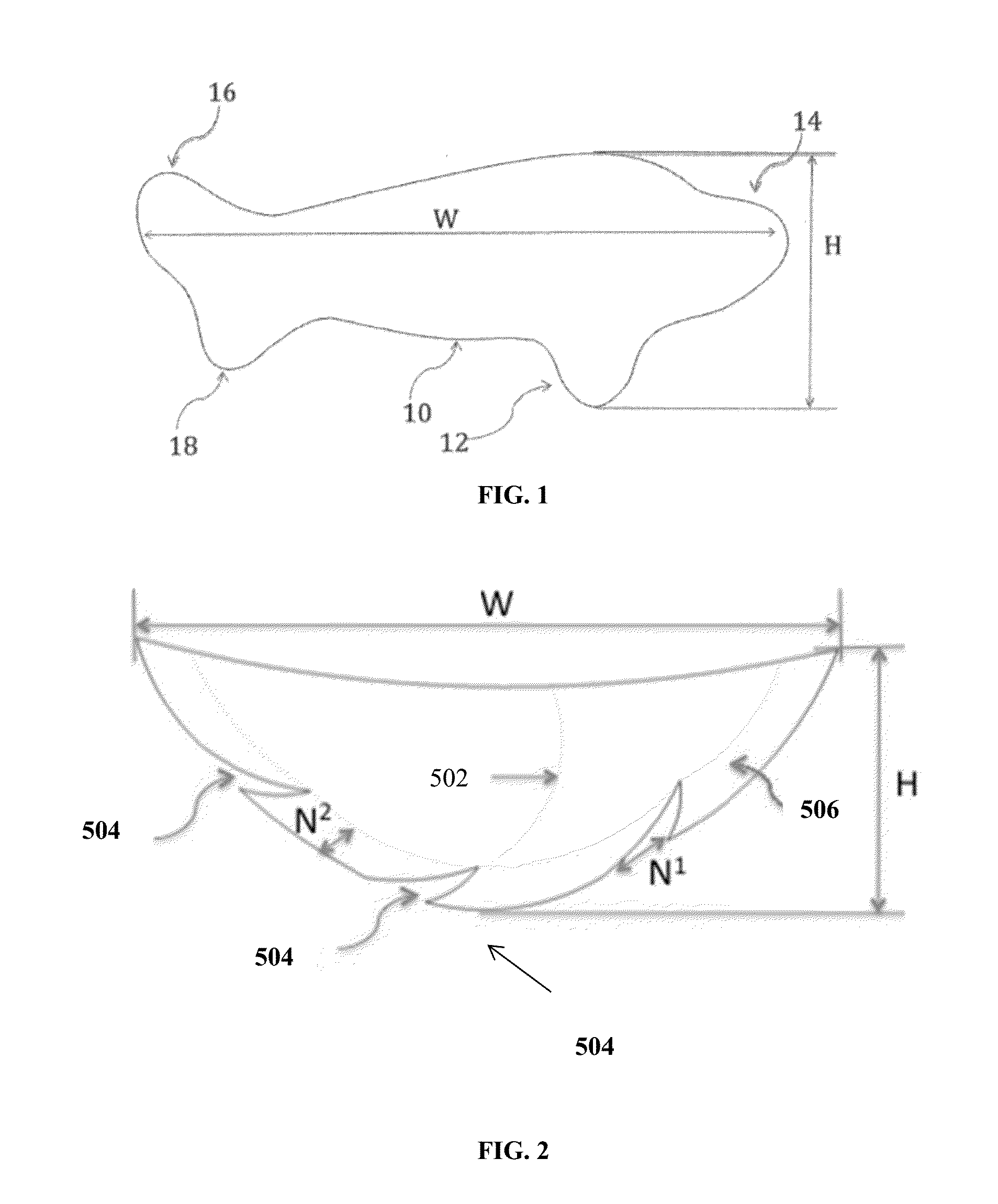
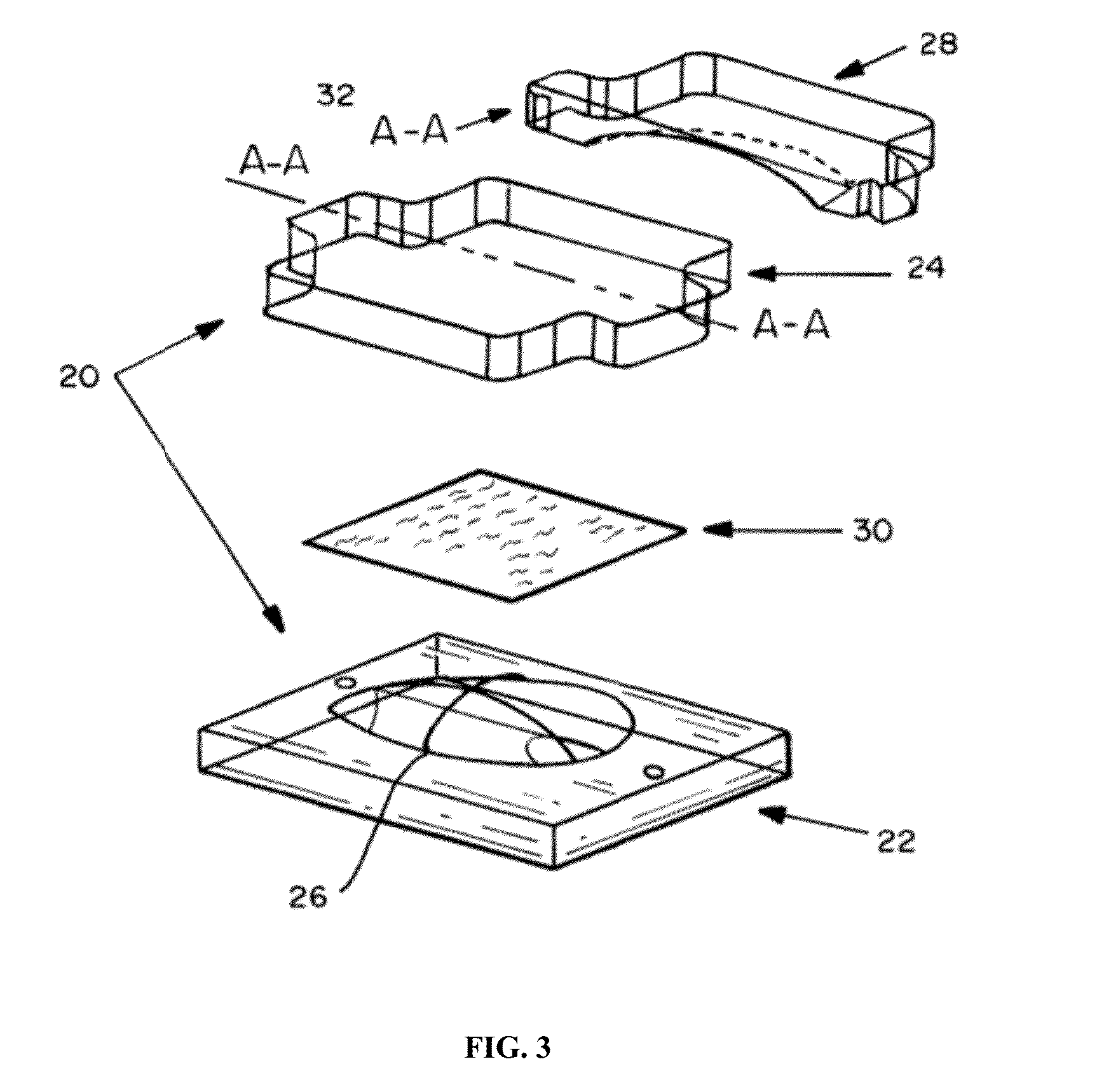
Absorbable implants for breast surgery that conform to the breast parenchyma and surrounding chest wall have been developed. These implants support newly lifted breast parenchyma, and / or a breast implant. The implants have mechanical properties sufficient to support a reconstructed breast, and allow the in-growth of tissue into the implant as it degrades. The implants have a strength retention profile allowing the support of the breast to be transitioned from the implant to regenerated host tissue, without significant loss of support. Three-dimensional implants for use in minimally invasive mastopexy / breast reconstruction procedures are also described, that confer shape to a patient's breast. These implants are self-reinforced, can be temporarily deformed, implanted in a suitably dissected tissue plane, and resume their preformed three-dimensional shape. The implants are preferably made from poly-4-hydroxybutyrate (P4HB) and copolymers thereof. The implants have suture pullout strengths that can resist the mechanical loads exerted on the reconstructed breast.
Owner:TEPHA INC
Absorbable implants for plastic surgery
ActiveUS9655715B2Sufficient mechanical propertyMinimization requirementsMammary implantsSurgeryMastopexyBreast reconstruction
Absorbable implants for breast surgery that conform to the breast parenchyma and surrounding chest wall have been developed. These implants support newly lifted breast parenchyma, and / or a breast implant. The implants have mechanical properties sufficient to support a reconstructed breast, and allow the in-growth of tissue into the implant as it degrades. The implants have a strength retention profile allowing the support of the breast to be transitioned from the implant to regenerated host tissue, without significant loss of support. Three-dimensional implants for use in minimally invasive mastopexy / breast reconstruction procedures are also described, that confer shape to a patient's breast. These implants are self-reinforced, can be temporarily deformed, implanted in a suitably dissected tissue plane, and resume their preformed three-dimensional shape. The implants are preferably made from poly-4-hydroxybutyrate (P4HB) and copolymers thereof. The implants have suture pullout strengths that can resist the mechanical loads exerted on the reconstructed breast.
Owner:TEPHA INC
Absorbable Implants for Plastic Surgery
ActiveUS20160022416A1Sufficient mechanical propertyMinimization requirementsMammary implantsSurgeryPullout strengthThree dimensional shape
Absorbable implants for breast surgery that conform to the breast parenchyma and surrounding chest wall have been developed. These implants support newly lifted breast parenchyma, and / or a breast implant. The implants have mechanical properties sufficient to support a reconstructed breast, and allow the ingrowth of tissue into the implant as it degrades. The implants have a strength retention profile allowing the support of the breast to be transitioned from the implant to regenerated host tissue, without significant loss of support. Three-dimensional implants for use in minimally invasive mastopexy / breast reconstruction procedures are also described, that confer shape to a patient's breast. These implants are self-reinforced, can be temporarily deformed, implanted in a suitably dissected tissue plane, and resume their preformed three-dimensional shape. The implants are preferably made from poly-4-hydroxybutyrate (P4HB) and copolymers thereof. The implants have suture pullout strengths that can resist the mechanical loads exerted on the reconstructed breast
Owner:TEPHA INC
Absorbable implants for plastic surgery
ActiveUS20150112434A1Solve the lack of mechanical propertiesMinimization requirementsMammary implantsArtificial flowers and garlandsMastopexyEngineering
Absorbable implants for breast surgery that conform to the breast parenchyma and surrounding chest wall have been developed. These implants support newly lifted breast parenchyma, and / or a breast implant. The implants have mechanical properties sufficient to support a reconstructed breast, and allow the in-growth of tissue into the implant as it degrades. The implants have a strength retention profile allowing the support of the breast to be transitioned from the implant to regenerated host tissue, without significant loss of support. Three-dimensional implants for use in minimally invasive mastopexy / breast reconstruction procedures are also described, that confer shape to a patient's breast. These implants are self-reinforced, can be temporarily deformed, implanted in a suitably dissected tissue plane, and resume their preformed three-dimensional shape. The implants are preferably made from poly-4-hydroxybutyrate (P4HB) and copolymers thereof. The implants have suture pullout strengths that can resist the mechanical loads exerted on the reconstructed breast.
Owner:TEPHA INC
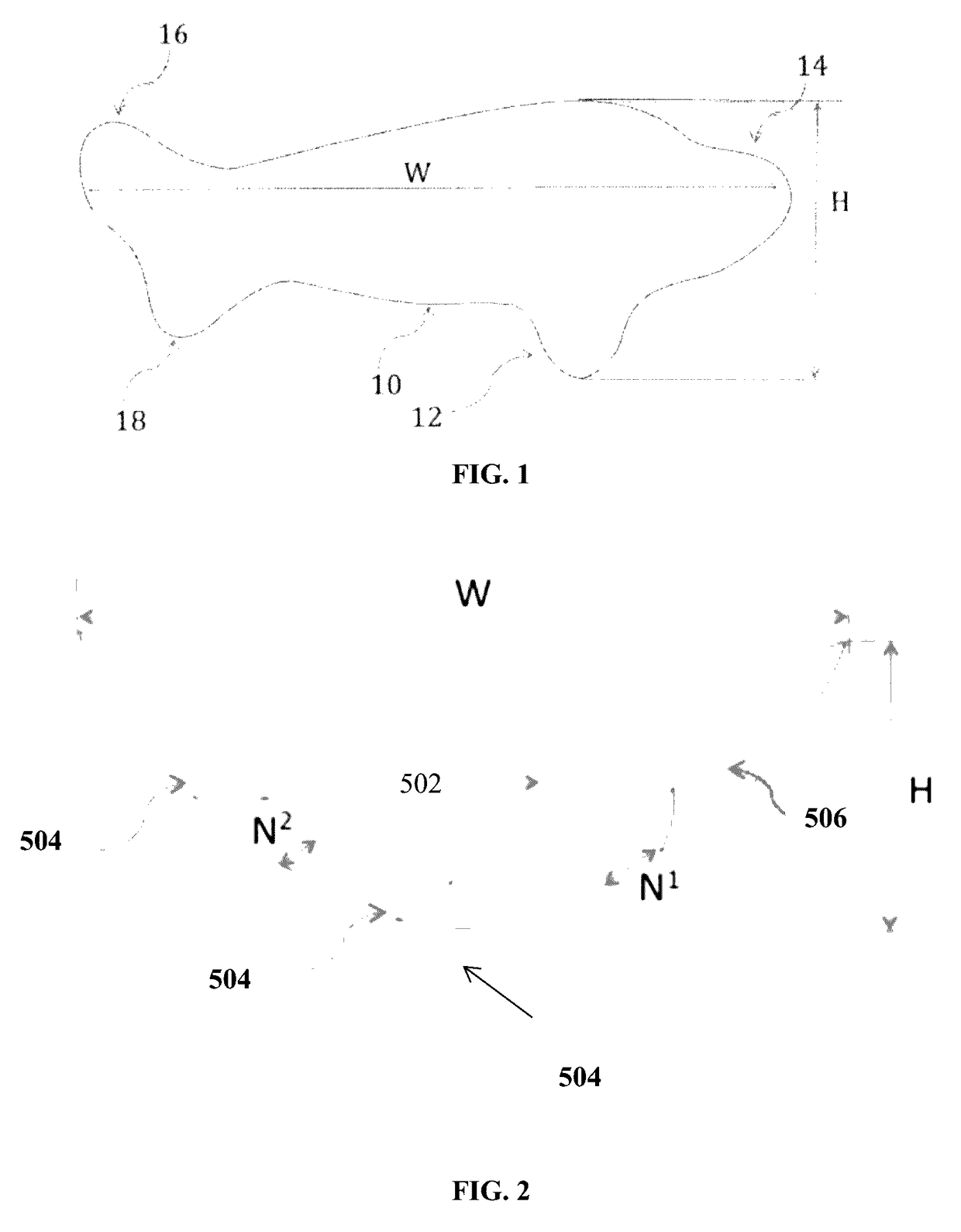
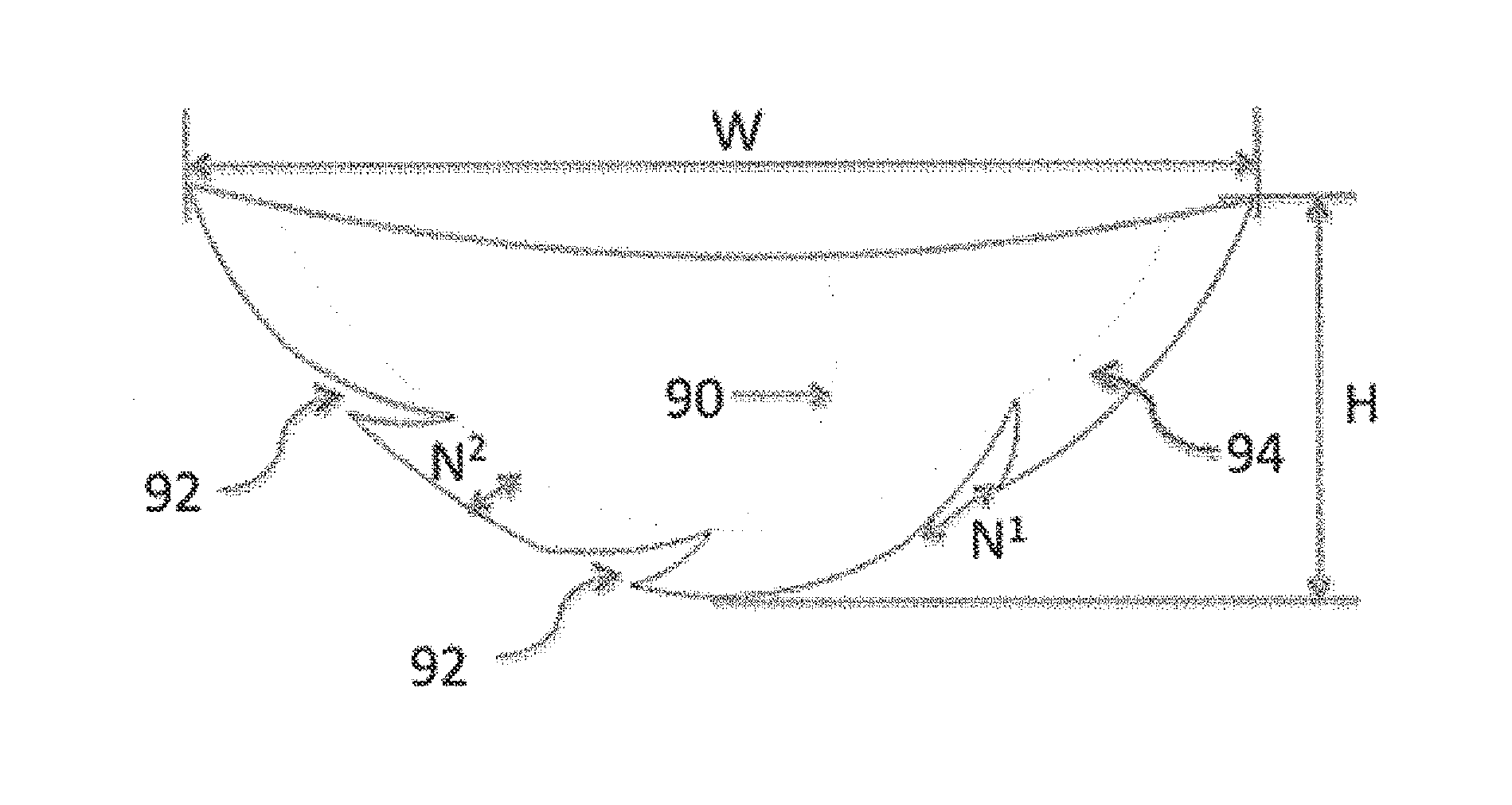
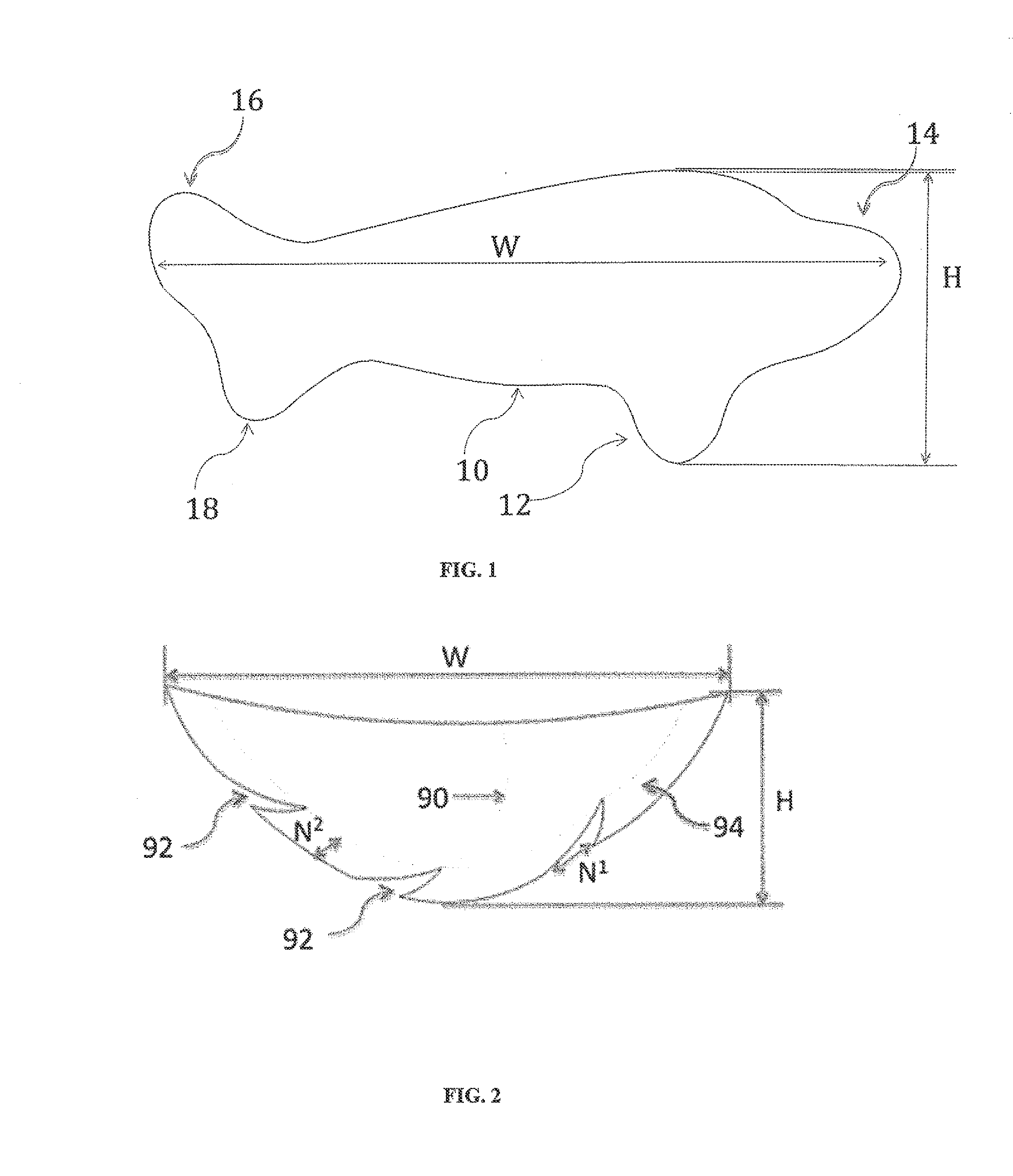
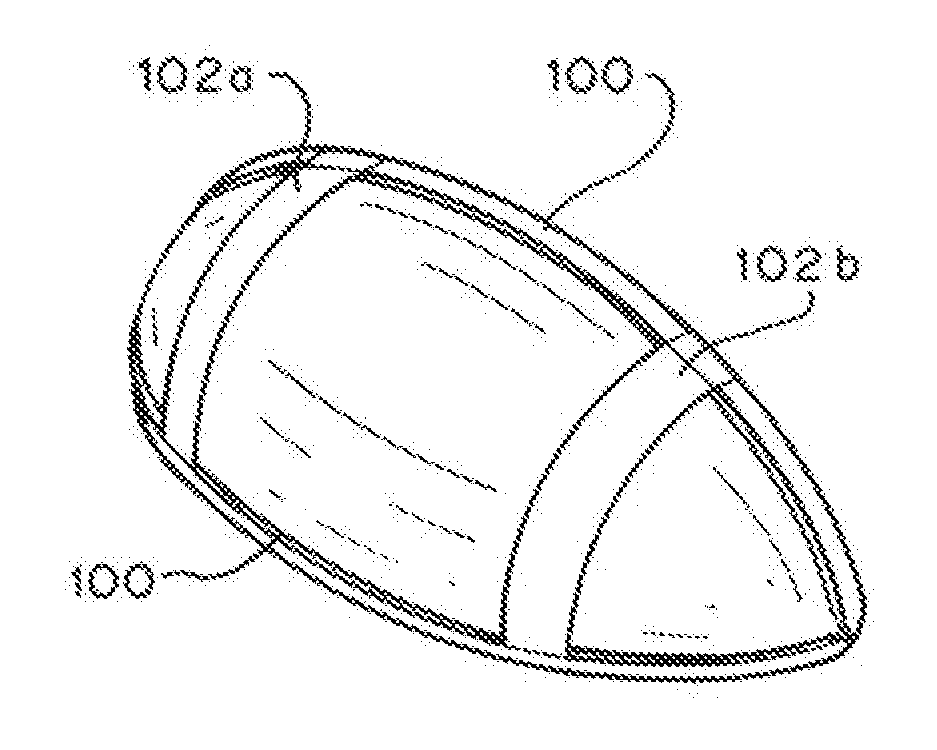
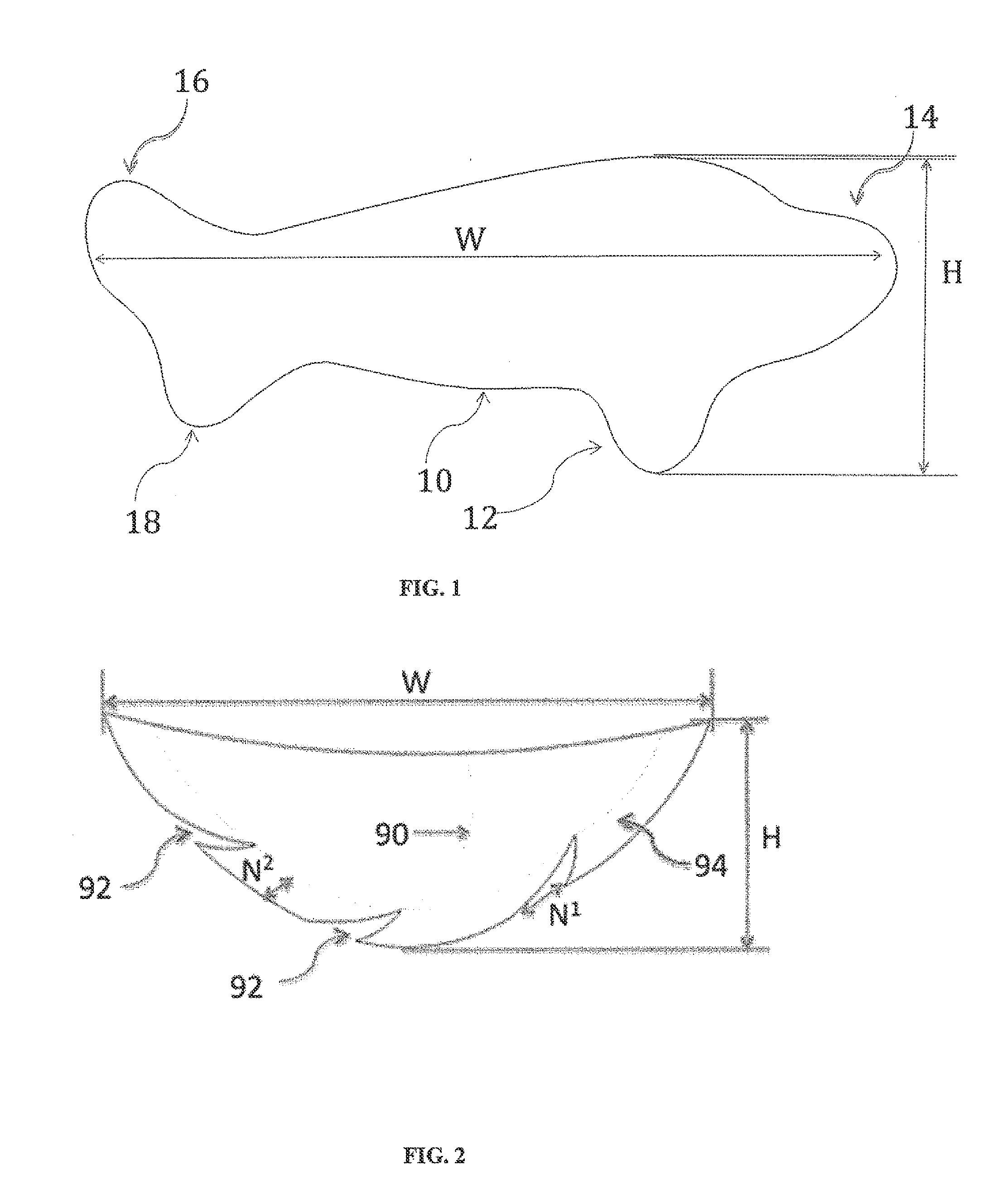
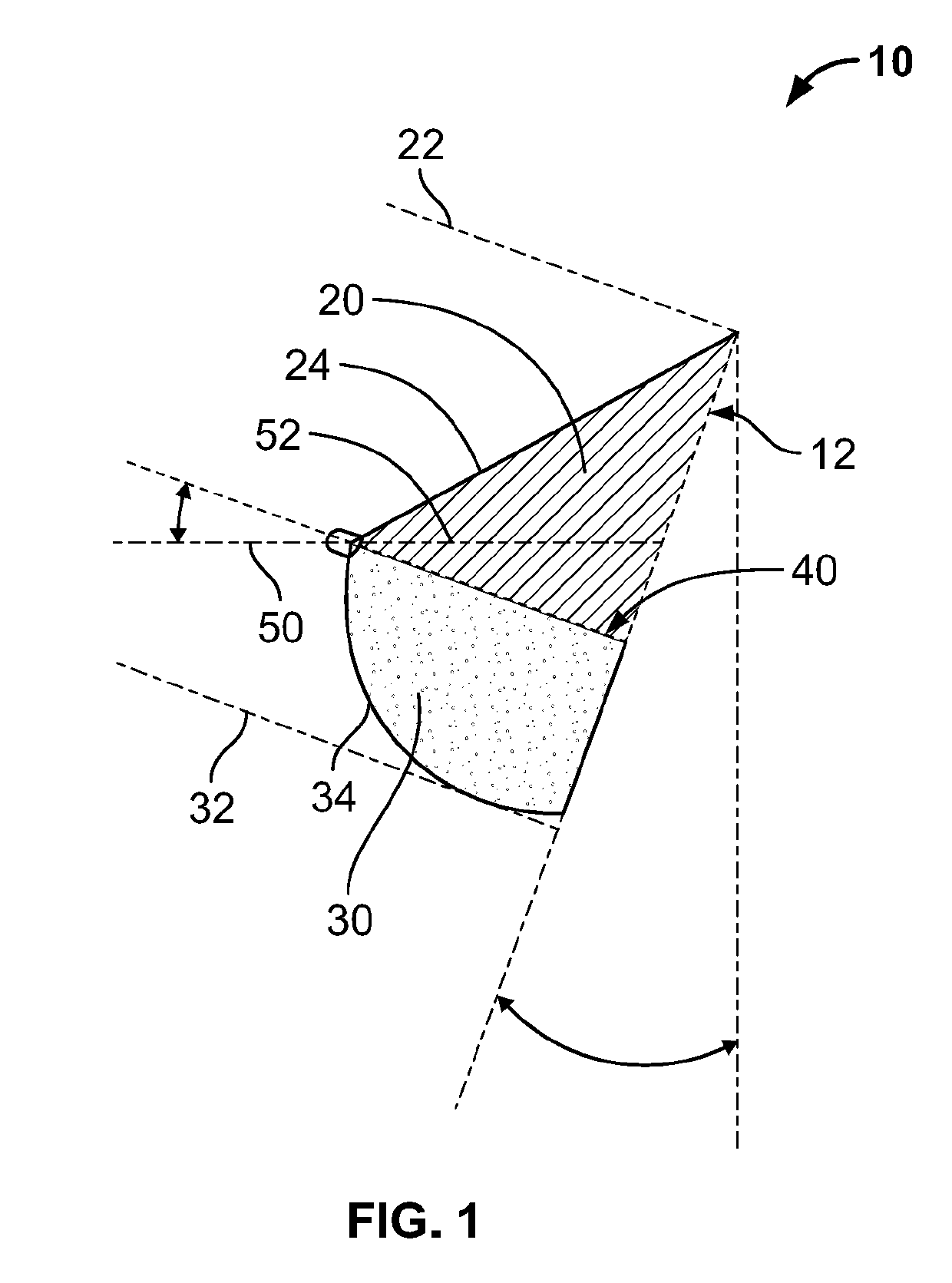
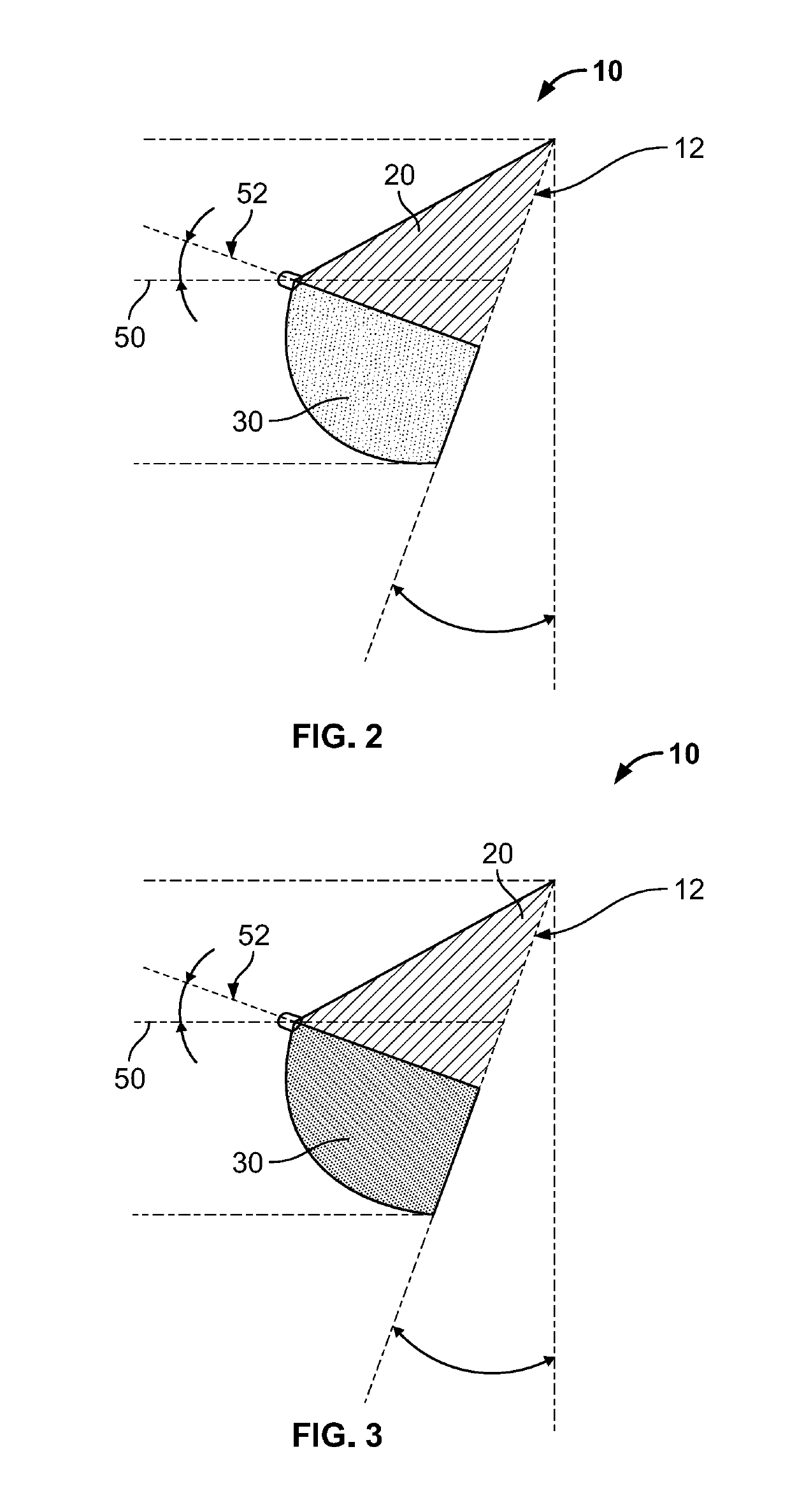
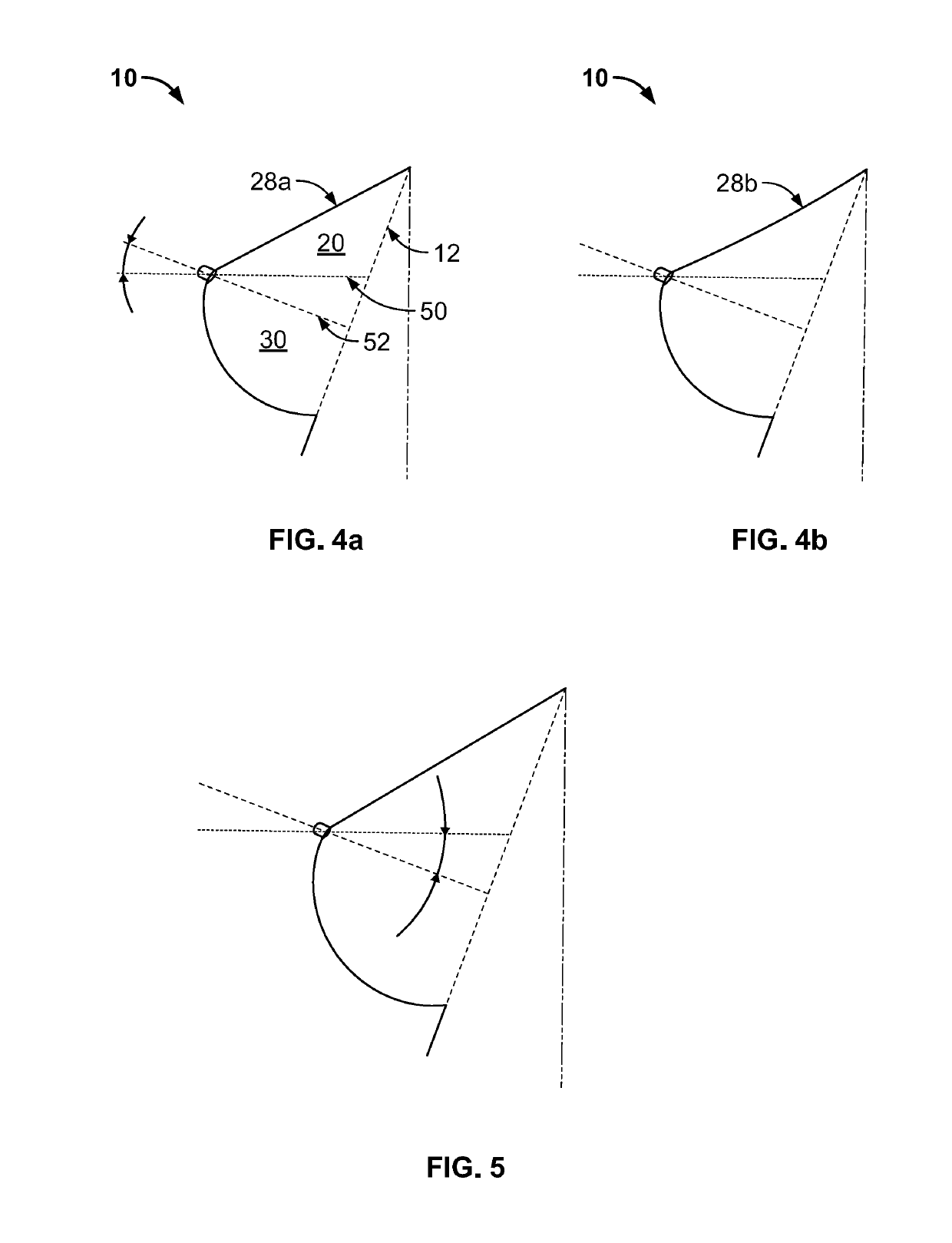
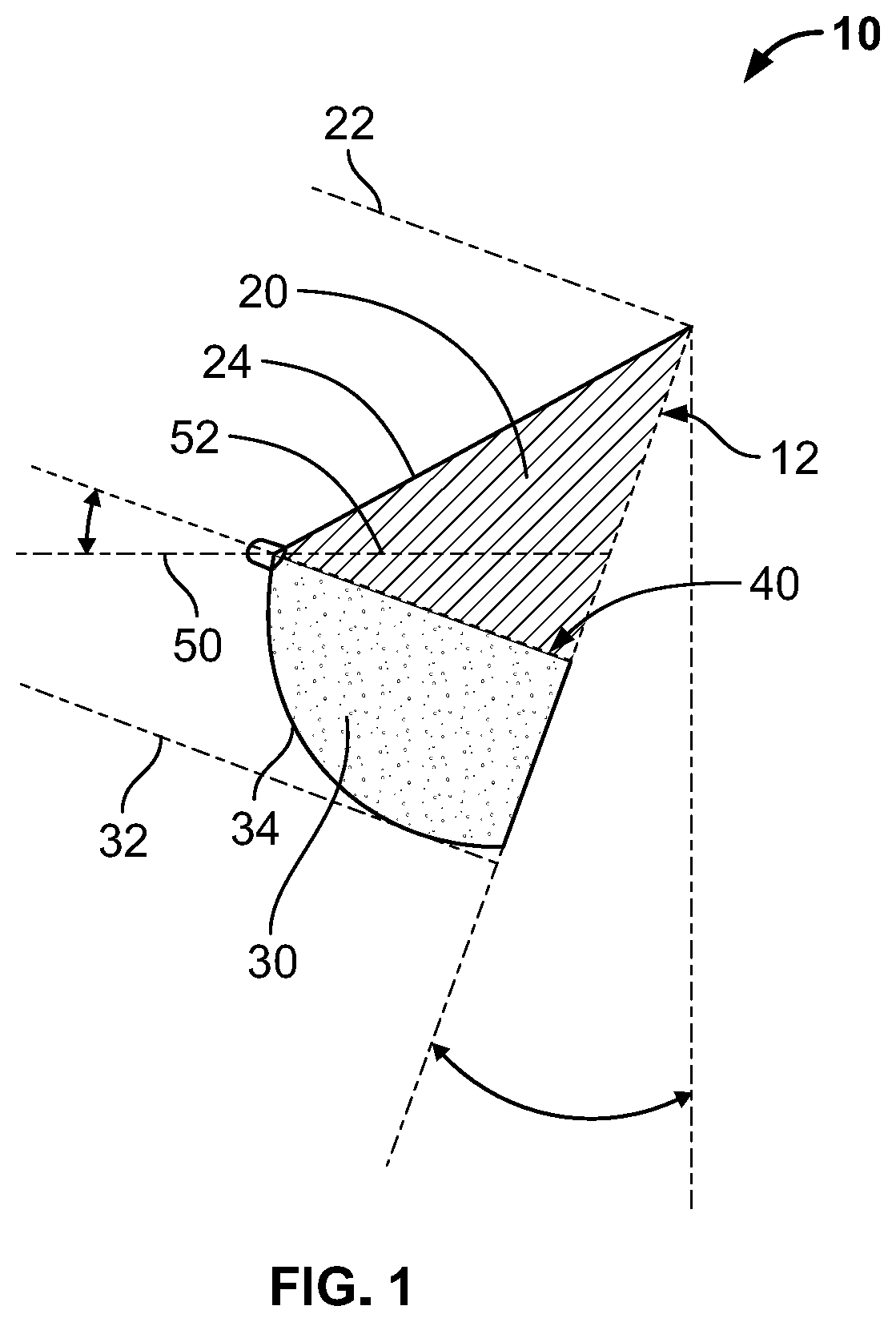
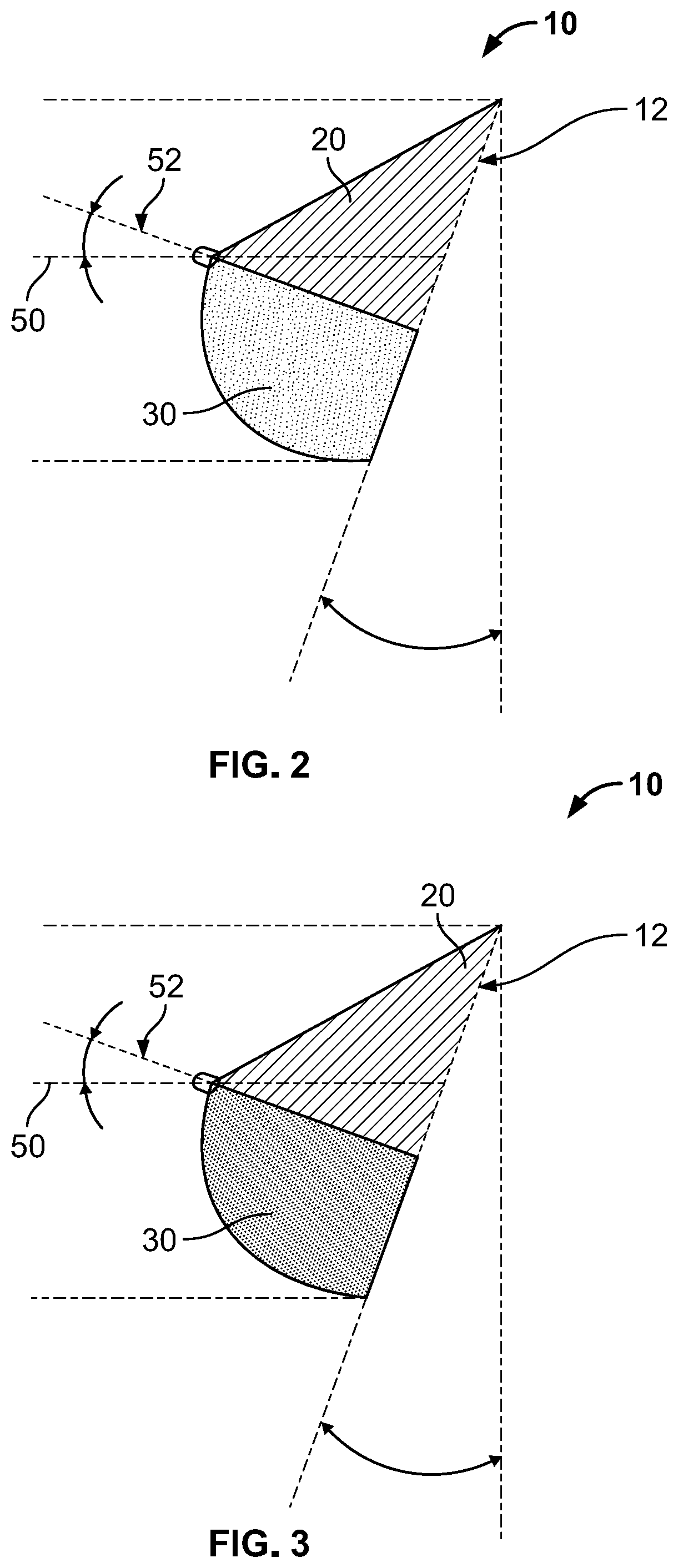
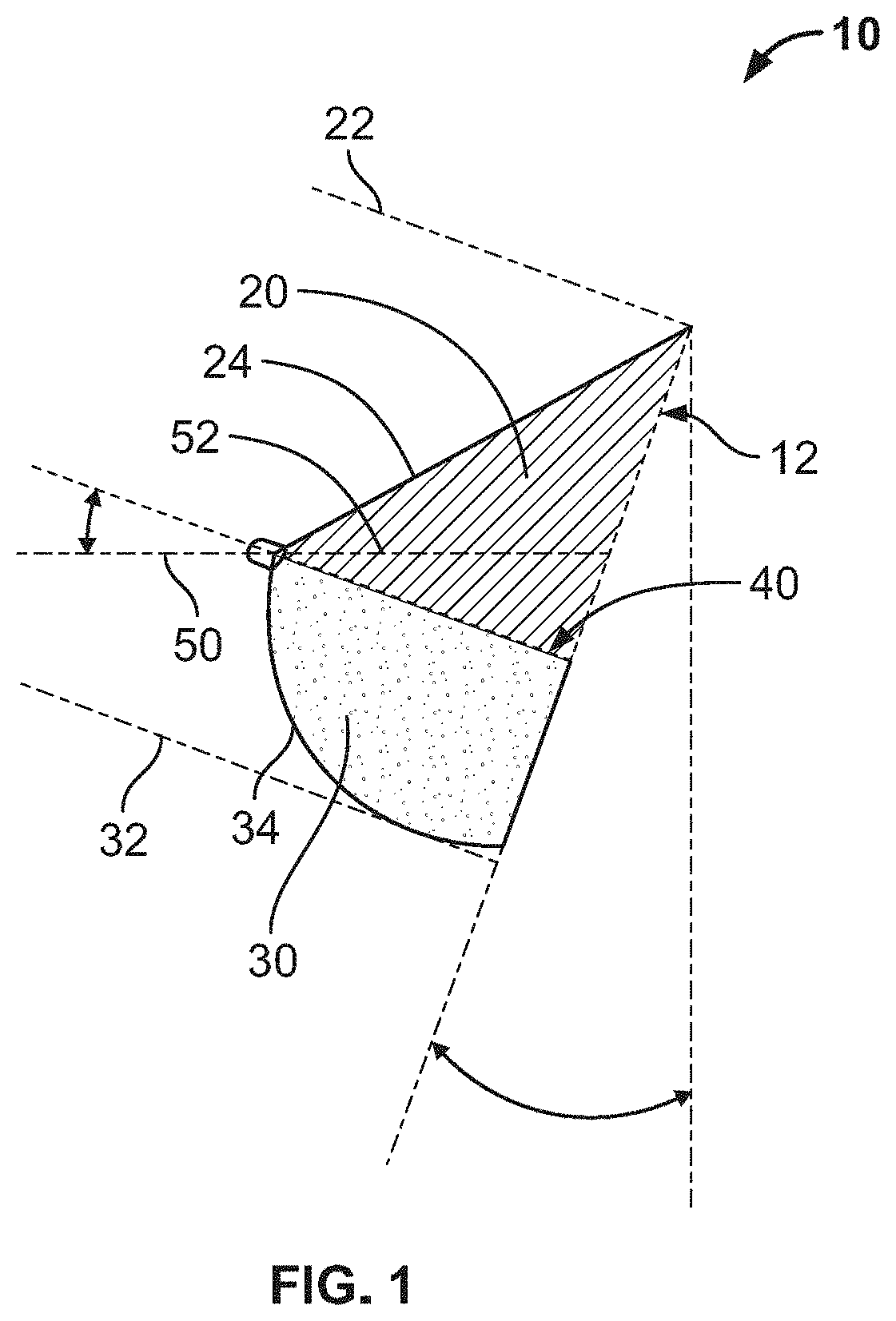
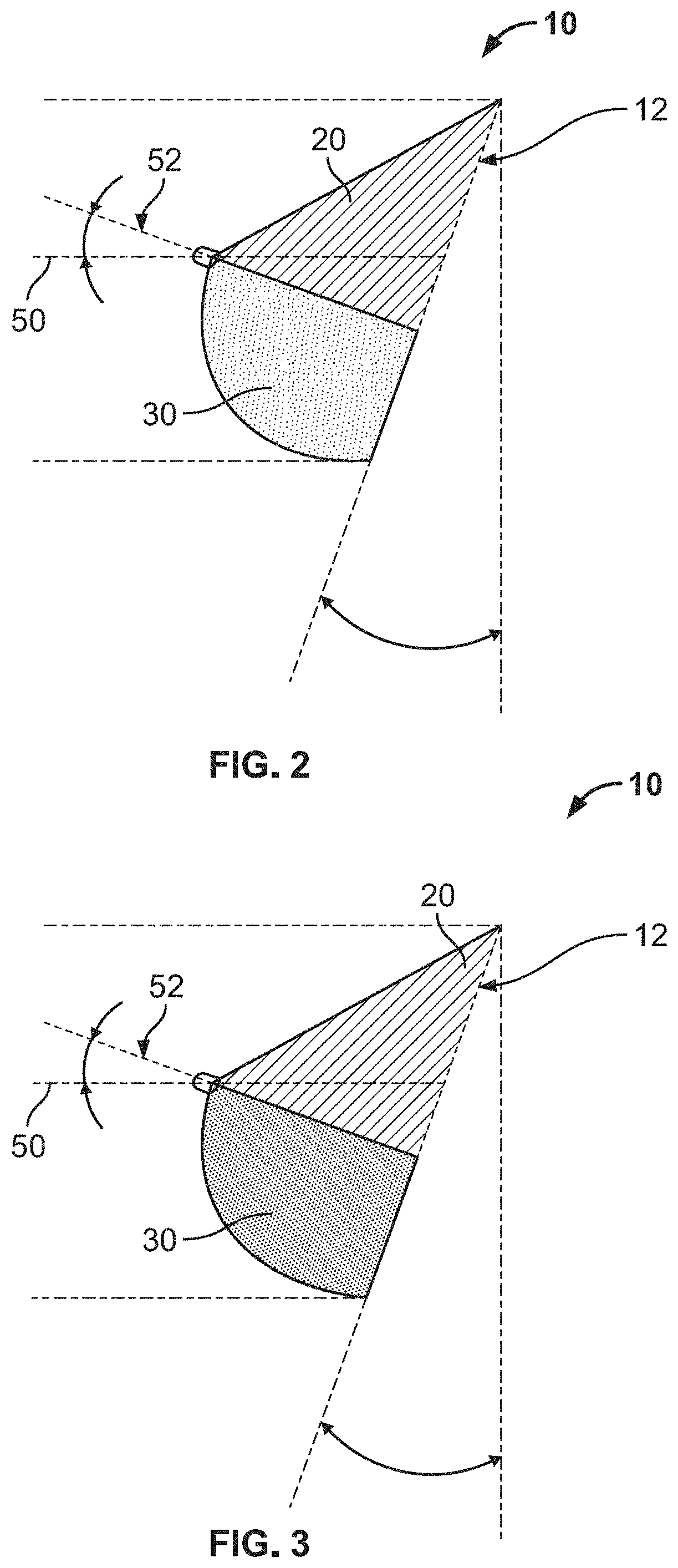
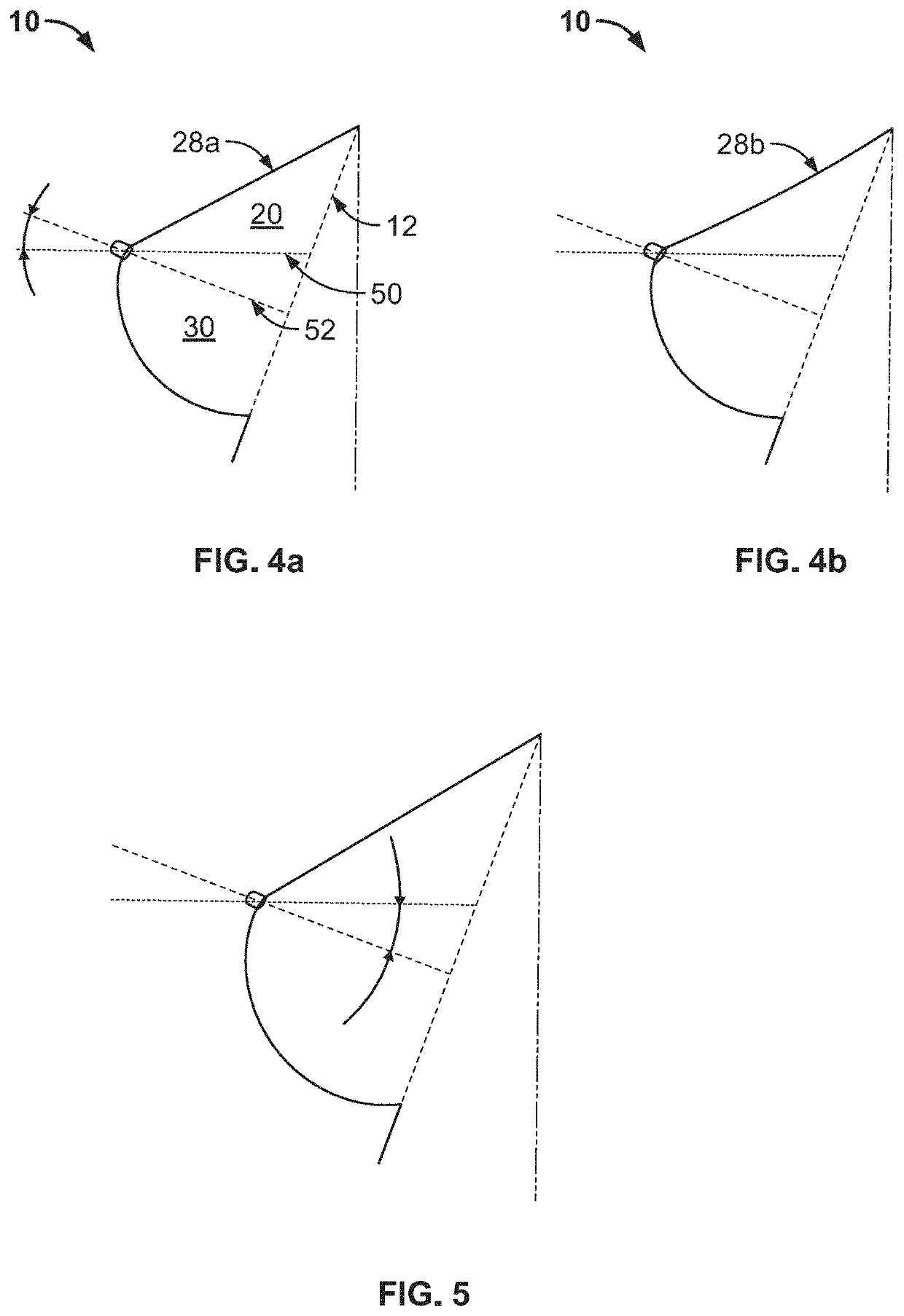
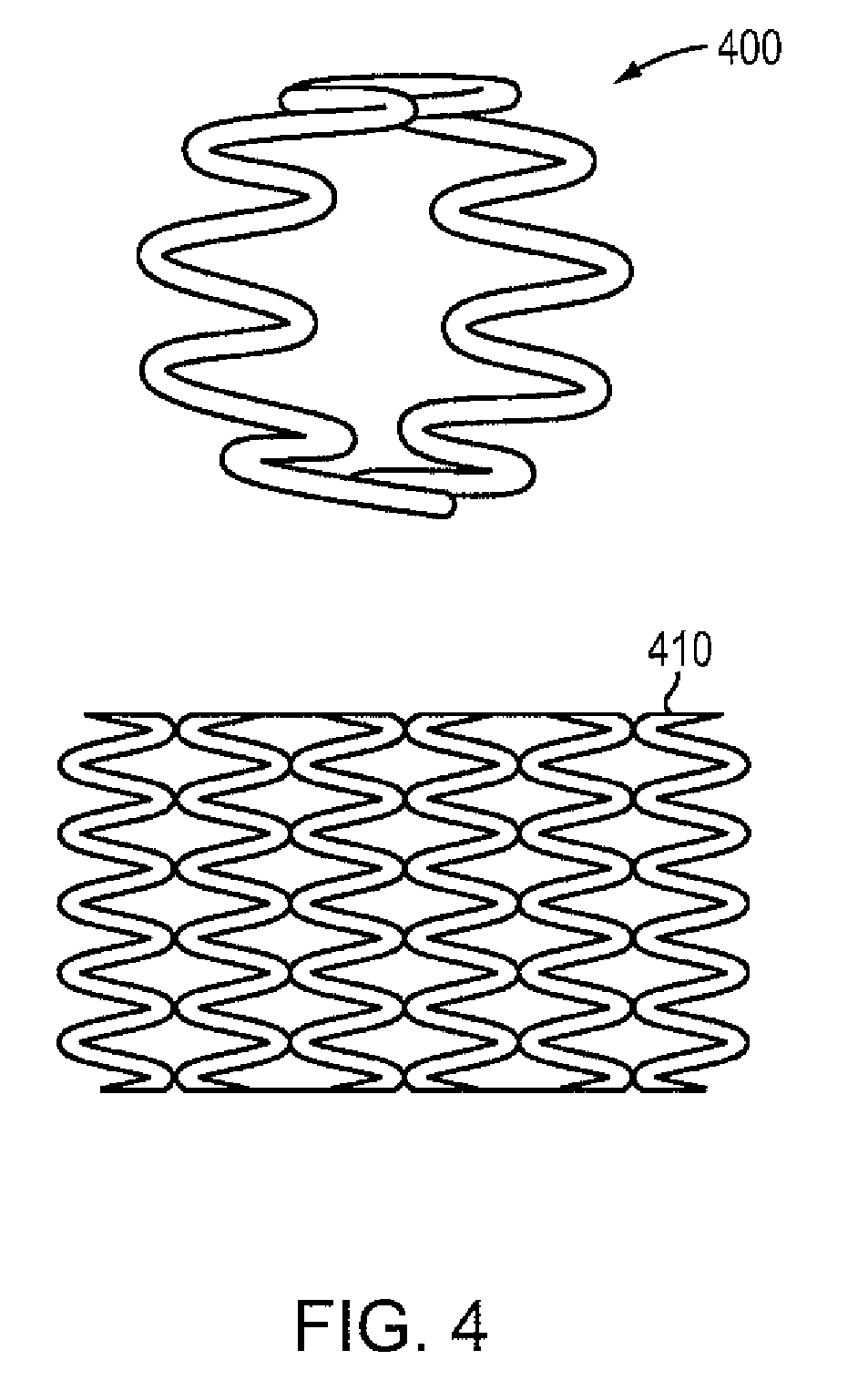
Full contour breast implant
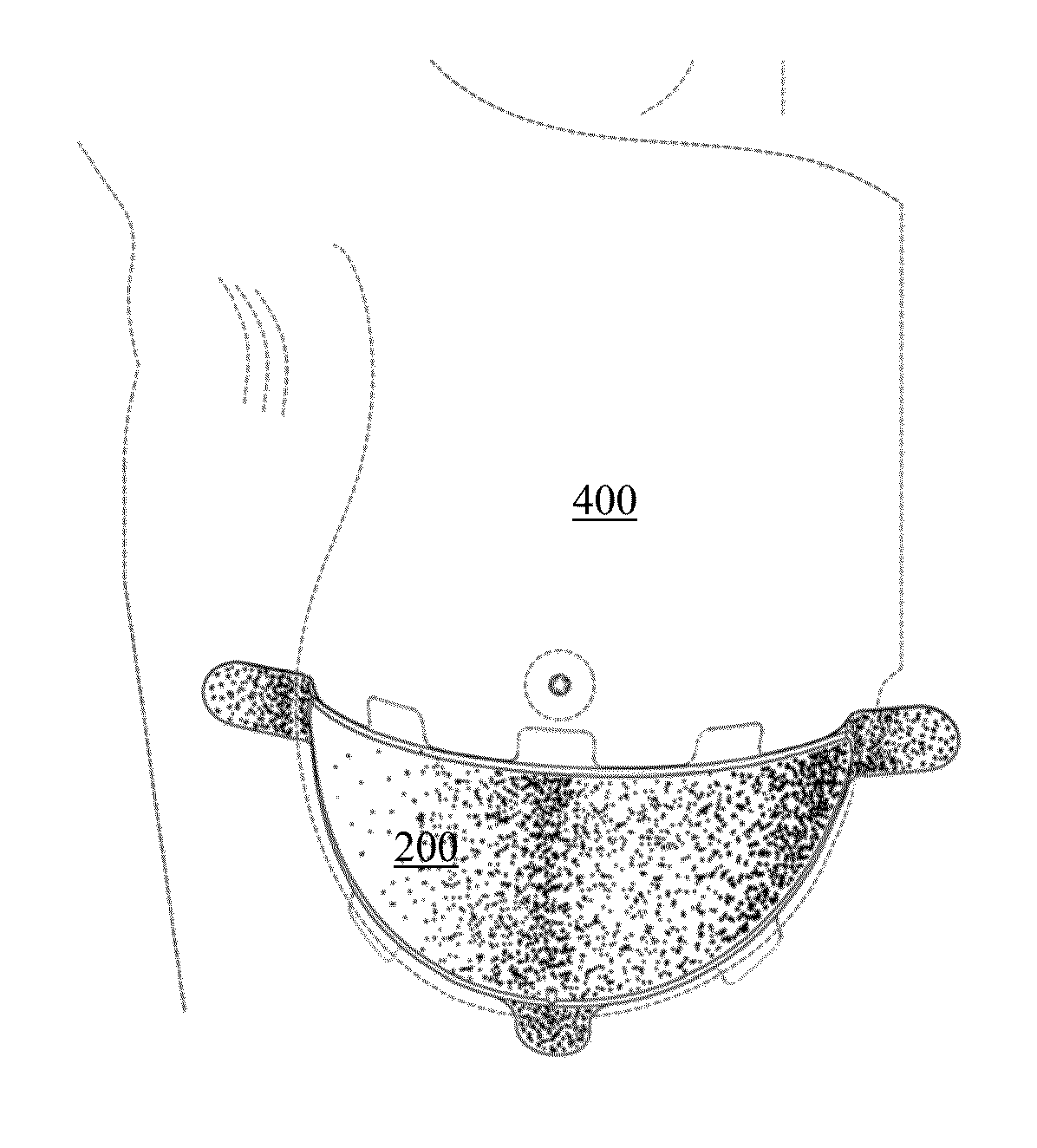
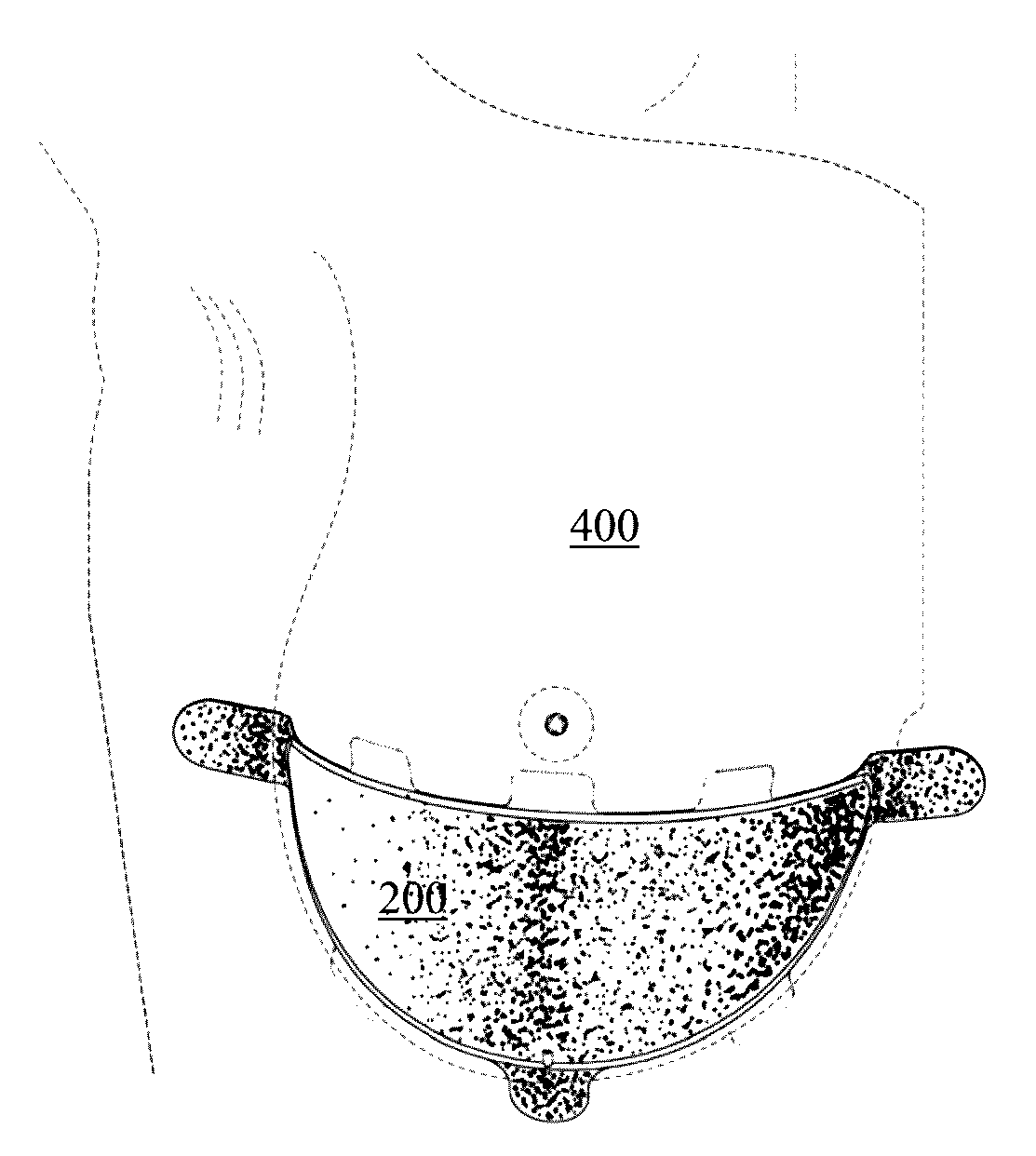
Full contour absorbable implants for breast surgery redistribute breast volume between the breast's upper and lower poles in exact and desirable ratios. The implants preferably redistribute breast volume so that the upper pole breast volume is 20-40% of the total volume, and the lower pole breast volume is 60-80% of the total volume. The implants are also designed to provide specific curvatures to the poles of the breast, and to angulate the nipple areolar complex slightly skyward so that the patient's nipple is positioned at an angle above the nipple meridian reference line. The implants are designed to be transitory, with sufficient strength retention to allow transition from support of the breast by the implant to support by regenerated host tissue growing in and around the implants, without any significant loss of support during or subsequent to remodeling. The implants may optionally be used with permanent breast implants.
Owner:TEPHA INC
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS7955616B2Lower potentialMinimally inhibit osteogenesis and subsequent bone healingSurgical adhesivesSkeletal disorderPyrrolidinonesCarboxylic acid
The present application is directed to compositions and methods for mechanically controlling the bleeding of bone. The compositions comprise in intimate admixture the following Components 1, 2 and 3: (1) a finely powdered, carboxylic acid salt comprising a carboxylate anion and a metallic cation, (2) a composition comprising pyrrolidone or an N-alkyl pyrrolidone wherein alkyl is a C1-C12 alkyl radical and an optional, biocompatible liquefying agent if the composition is in solid form at room temperature, and (3) an optional analgesic, wherein the analgesic is present in a free base and salt form.
Owner:ABYRX
Expandable absorbable implants for breast reconstruction and augmentation
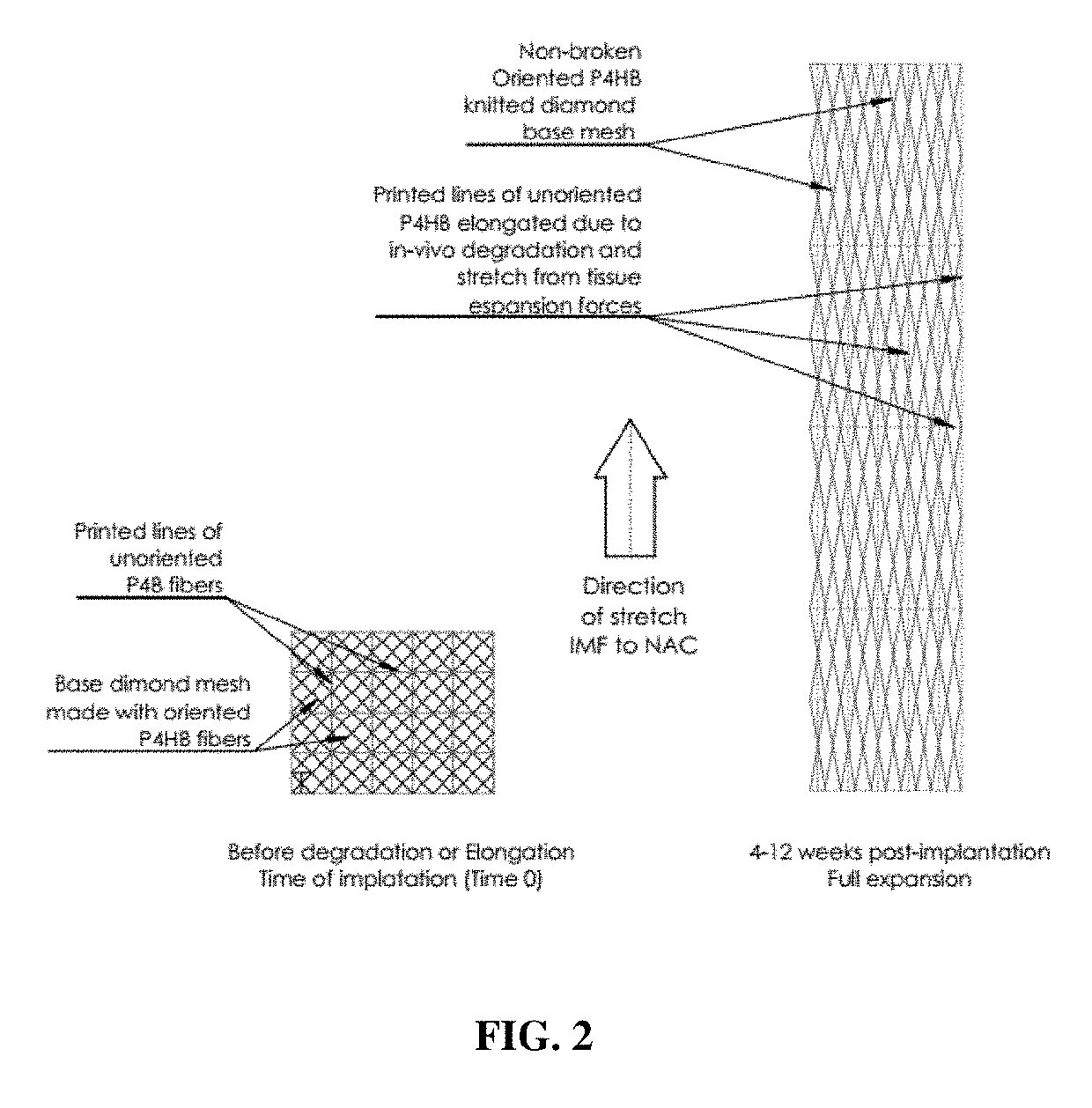
ActiveUS20190254807A1Relieve pressureDecreases short-termMammary implantsDiagnosticsBreast implantAbsorbable Implants
Expandable absorbable implants have been developed that are suitable for breast reconstruction following mastectomy. The implants can be implanted in the vicinity of a tissue expander, for example, by suturing to the detached edge of the pectoralis major muscle to function as a pectoralis extender, and used to form a sling for a tissue expander. The implants, which permit tissue-ingrowth and slowly degrade, can be expanded in the breast using a tissue expander in order to form a pocket for a permanent breast implant. After expansion, the tissue expander can be removed and replaced with a permanent breast implant. The expandable implants help reduce patient discomfort resulting from tissue expansion, and avoid the need to use allografts or xenografts to create the pocket for the tissue expander. The expandable absorbable implant preferably comprises poly-4-hydroxybutyrate or copolymer thereof.
Owner:TEPHA INC
Full contour breast implant
ActiveUS11154393B2Easy to appreciateNice appearanceMammary implantsNipple areola complexBreast implant
Full contour absorbable implants for breast surgery redistribute breast volume between the breast's upper and lower poles in exact and desirable ratios. The implants preferably redistribute breast volume so that the upper pole breast volume is 20-40% of the total volume, and the lower pole breast volume is 60-80% of the total volume. The implants are also designed to provide specific curvatures to the poles of the breast, and to angulate the nipple areolar complex slightly skyward so that the patient's nipple is positioned at an angle above the nipple meridian reference line. The implants are designed to be transitory, with sufficient strength retention to allow transition from support of the breast by the implant to support by regenerated host tissue growing in and around the implants, without any significant loss of support during or subsequent to remodeling. The implants may optionally be used with permanent breast implants.
Owner:TEPHA INC
Absorbable Implants and Methods for Their Use in Hemostasis
InactiveUS20120027817A1Lower potentialMinimally inhibit osteogenesis and subsequent bone healingBiocideSurgical adhesivesPolymer scienceAbsorbable Implants
Owner:ORTHOCON INC
Absorbable medical element suitable for insertion into the body, in particular an absorbable implant
InactiveUS20070016307A1Heal fastOptimisation of cell adsorptionJoint implantsTissue cultureFree zoneAbsorbable Implants
Owner:GFE MEDIZINTECHN
Absorbable medical element suitable for insertion into the body, in particular an absorbable implant
InactiveCN1895686AHeal fastImprove adsorption capacitySurgeryLiquid/solution decomposition chemical coatingFree zoneAbsorbable Implants
Owner:GFE MEDIZINTECHN
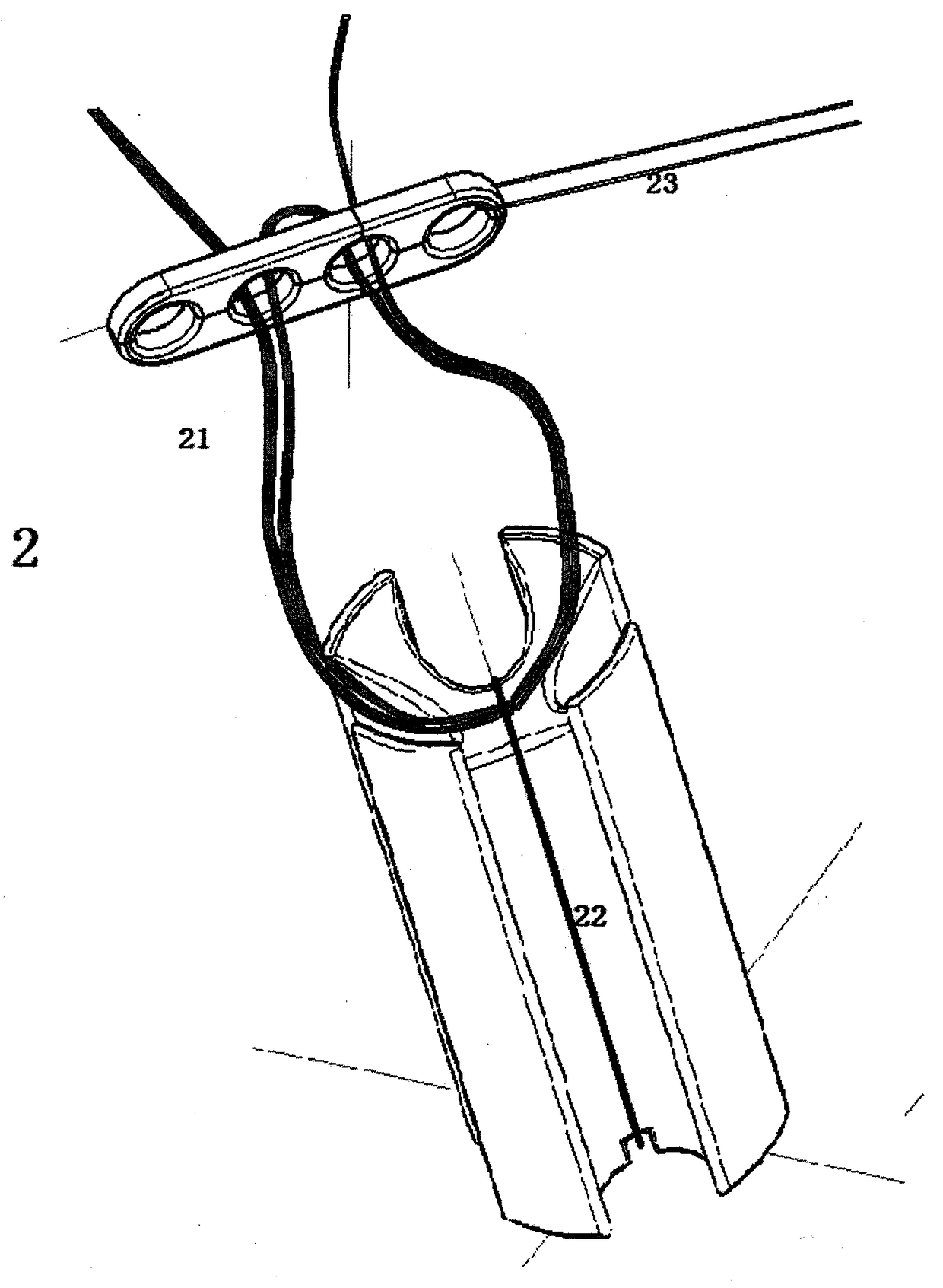
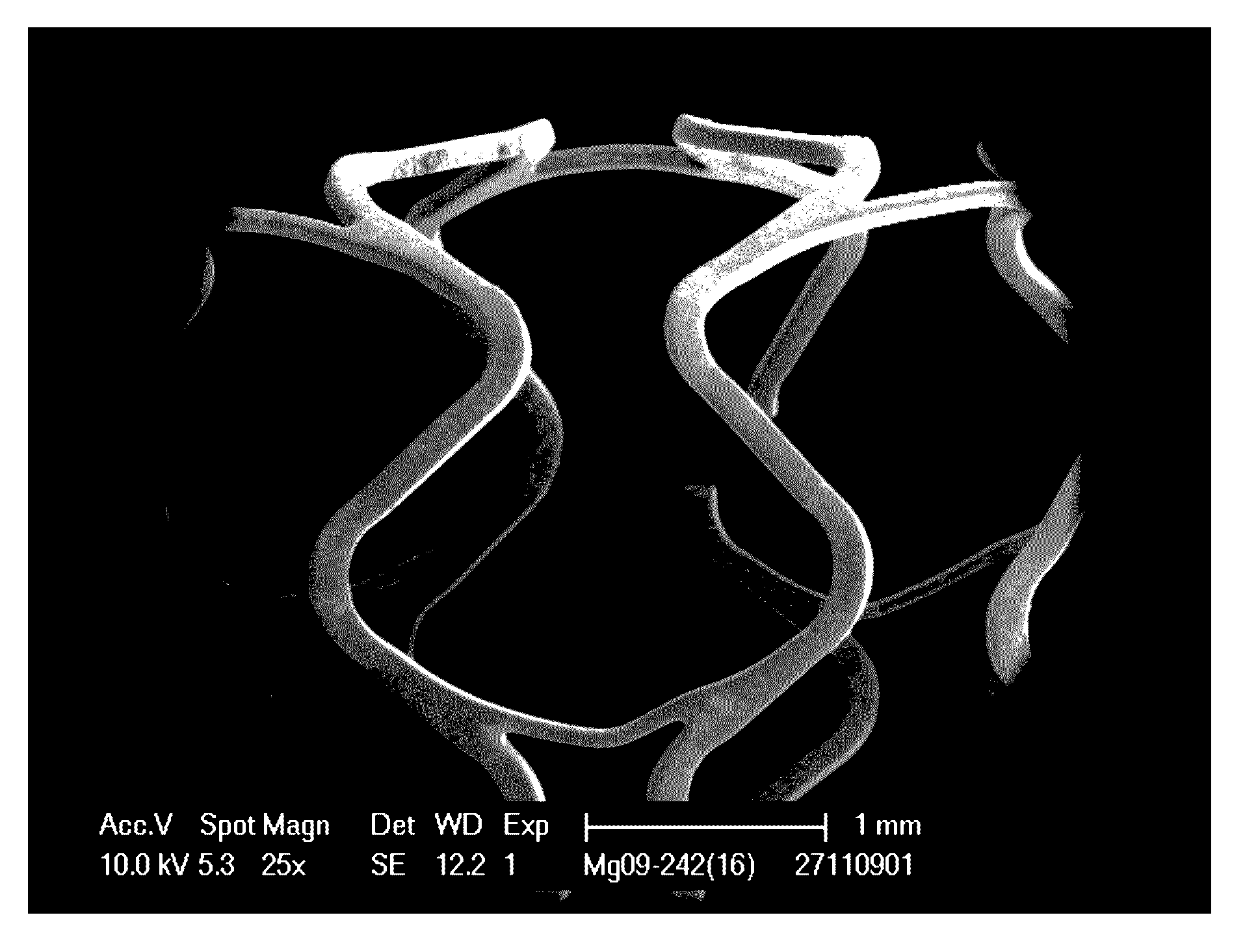
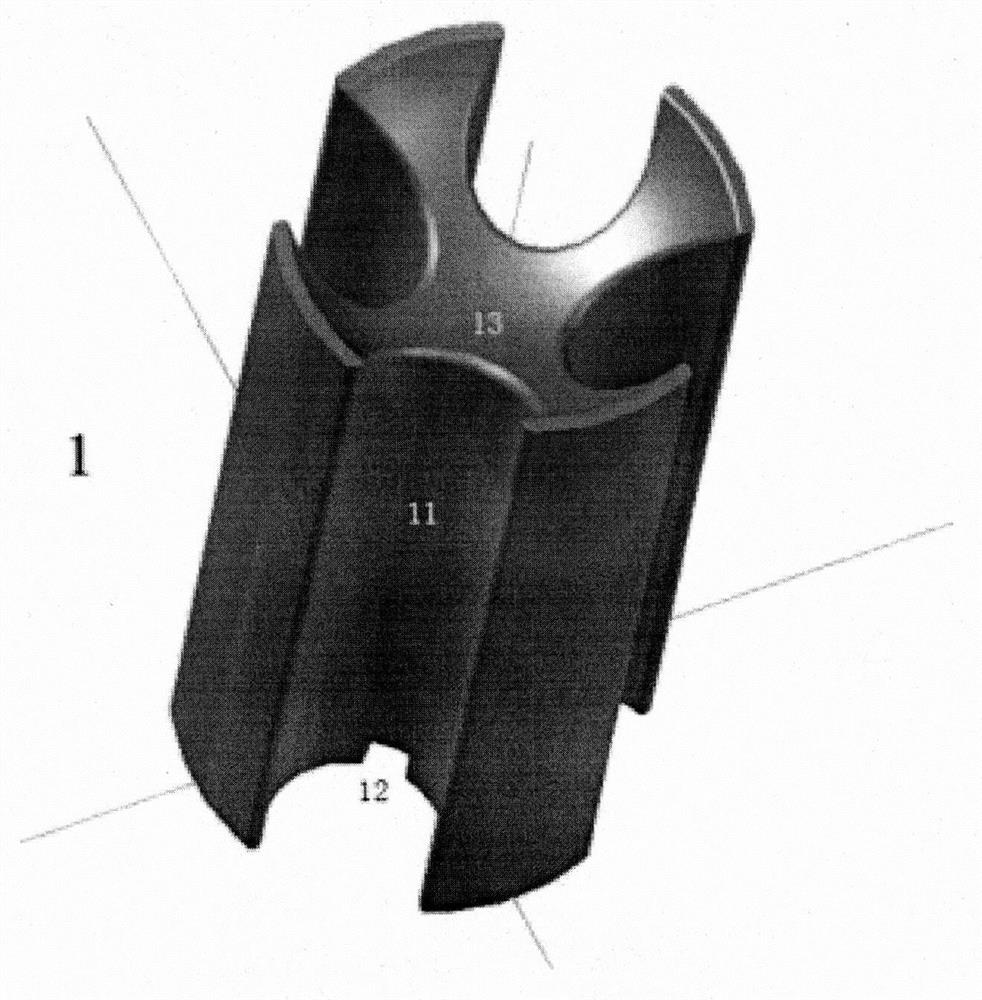
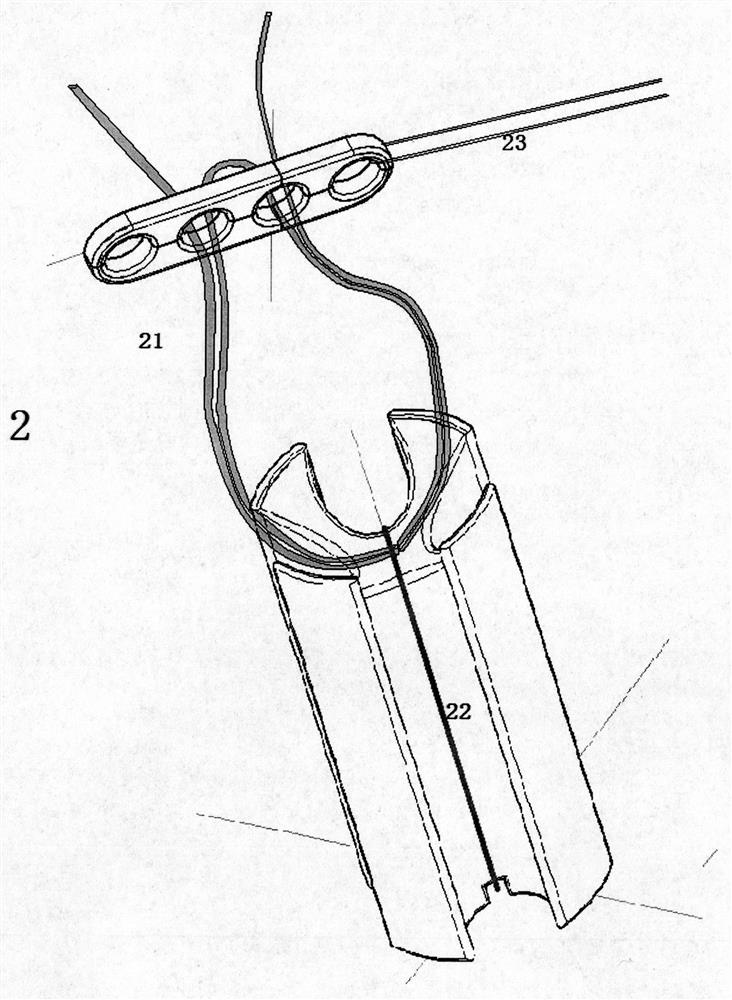
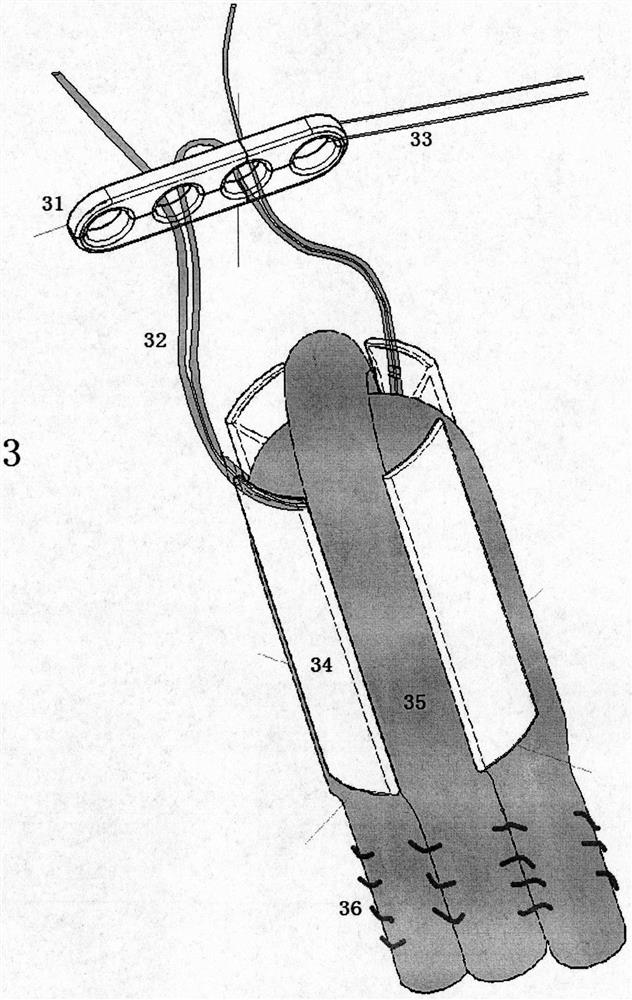
Ligament fixation system comprising suspensory steel plate and absorbable implant
ActiveCN108209990AReduce shear stressEasy to handleSuture equipmentsStaplesSynovial fluidFluid joints
The invention relates to a ligament fixation system comprising a suspensory steel plate and an absorbable implant and an application method of the system. The ligament fixation system includes the suspensory steel plate, the absorbable implant, a suspensory thread and a suture. The implant is in a cylindrical shape, parallel open pore passages are symmetrically distributed in the periphery of theimplant, an arc-shaped groove smoothly connected with a ligament graft is formed in the top of the implant, and a square groove for facilitating suture fixation is formed in the bottom of the implant.The implant makes the contact area of the ligament graft and the surface of a bone tunnel increased, prevents synovial fluid from permeating into the bone tunnel, effectively reduces the occurrence of a 'windshield wiper' effect, and prevents friction and breakage of the ligament graft. The application of the absorbable material is beneficial to achieving true tendon-bone healing. The ligament fixation system has wide raw material sources, and is convenient to manufacture, fast to assemble and easy to use, the tendon processing steps can be simplified, the operation time can be shortened, andthe firmness of the ligament graft can be significantly improved.
Owner:BEIJING WANJIE MEDICAL DEVICE CO LTD
Full contour breast implant
ActiveUS20220079741A1Easy to appreciateNice appearanceMammary implantsNipple areola complexBreast implant
Full contour absorbable implants for breast surgery redistribute breast volume between the breast's upper and lower poles in exact and desirable ratios. The implants preferably redistribute breast volume so that the upper pole breast volume is 20-40% of the total volume, and the lower pole breast volume is 60-80% of the total volume. The implants are also designed to provide specific curvatures to the poles of the breast, and to angulate the nipple areolar complex slightly skyward so that the patient's nipple is positioned at an angle above the nipple meridian reference line. The implants are designed to be transitory, with sufficient strength retention to allow transition from support of the breast by the implant to support by regenerated host tissue growing in and around the implants, without any significant loss of support during or subsequent to remodeling. The implants may optionally be used with permanent breast implants.
Owner:TEPHA INC
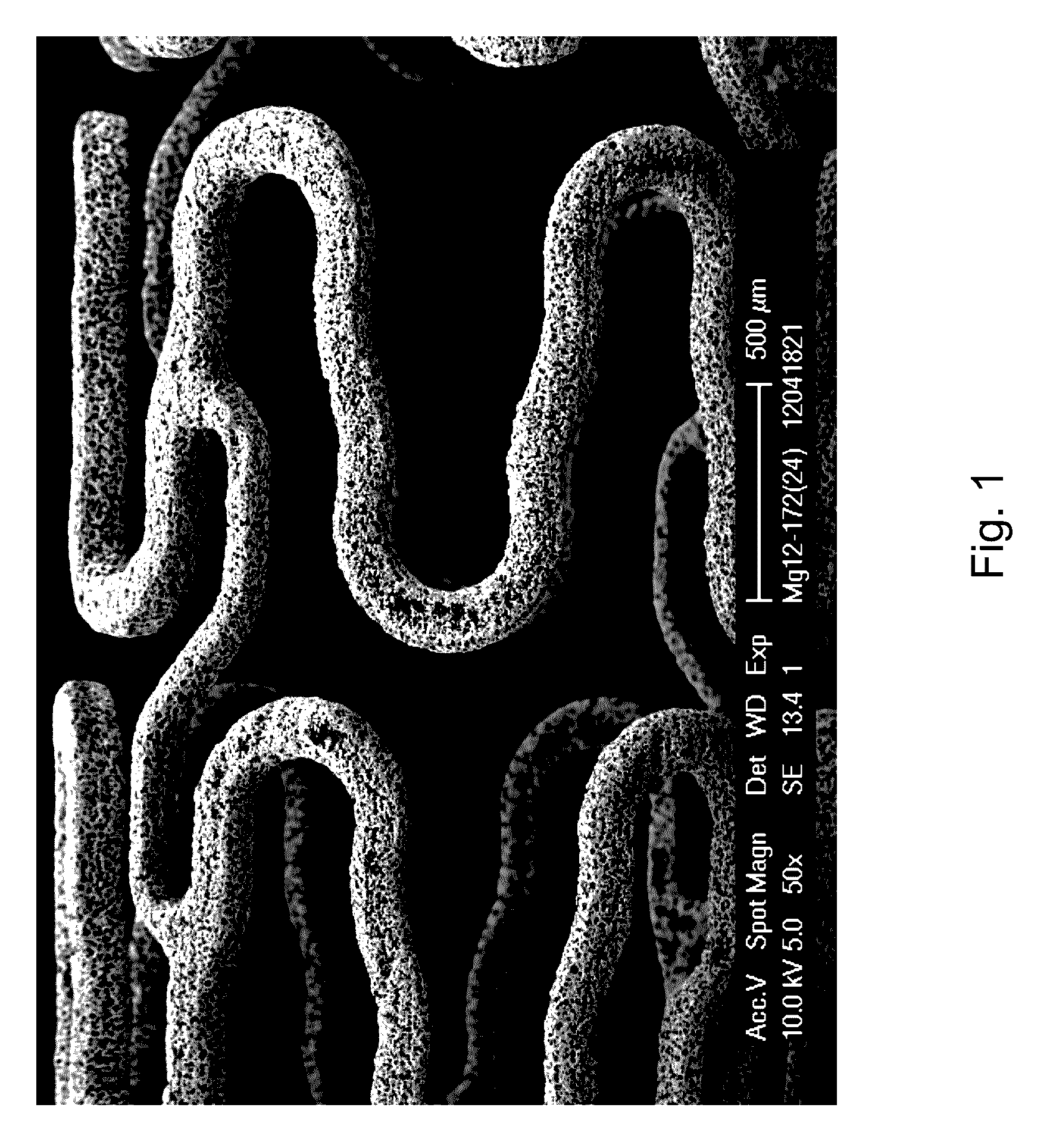
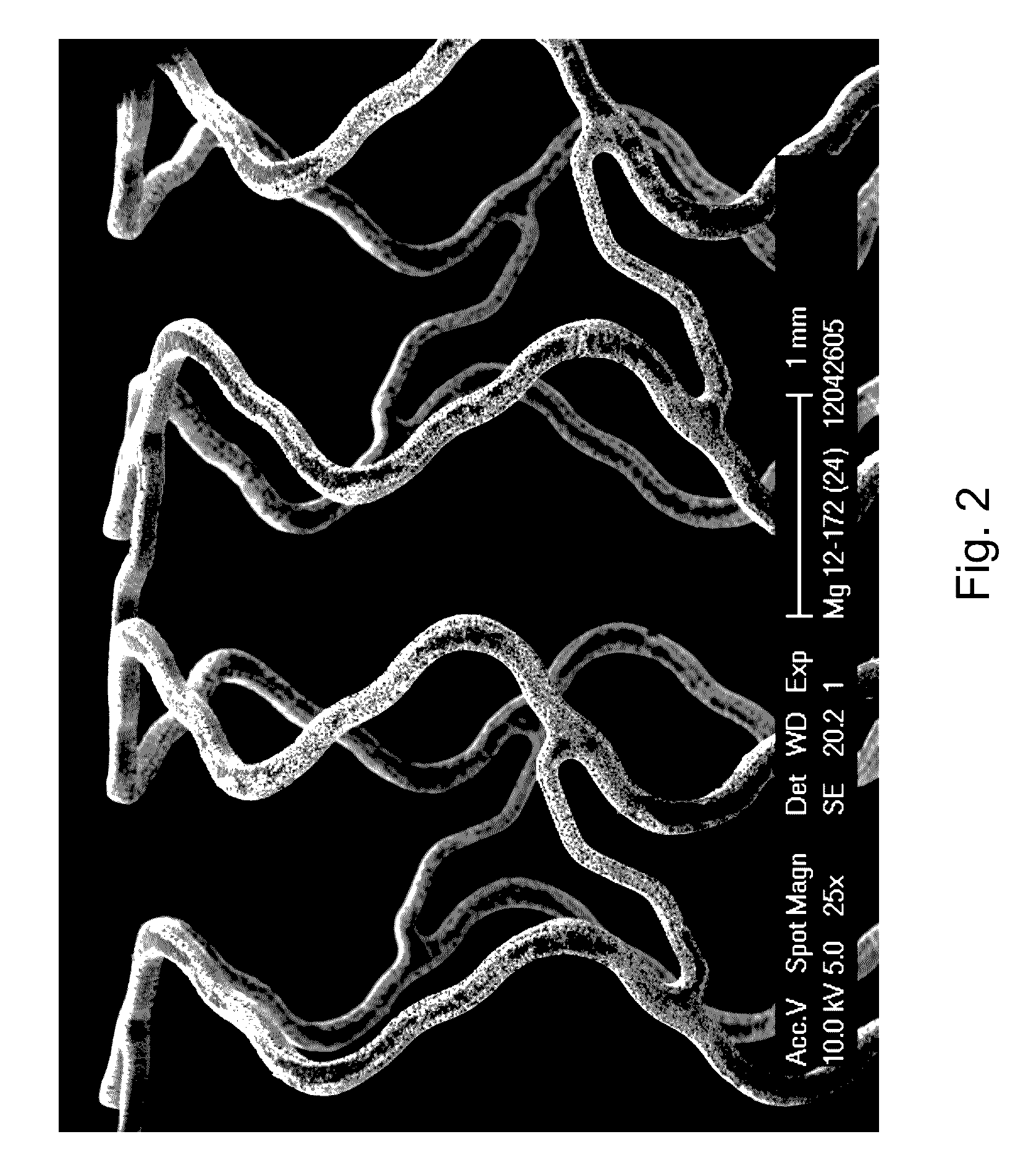
Magnesium-Based Absorbable Implants
An implantable tissue filler including a particulate material suspended in a carrier, the particulate material having more than about 70% by weight magnesium and about 2-20% by weight lithium, wherein the particulate material is substantially free of rare earth metals.
Owner:ZORION MEDICAL
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS8105628B2Lower potentialMinimally inhibit osteogenesis and subsequent bone healingBiocidePowder deliveryBarium saltTG - Triglyceride
Owner:ABYRX
Full contour breast implant
ActiveUS20220079742A1Easy to appreciateNice appearanceMammary implantsNipple areola complexBreast implant
Owner:TEPHA INC
Microstructured absorbable implant
Owner:BIOTRONIK AG
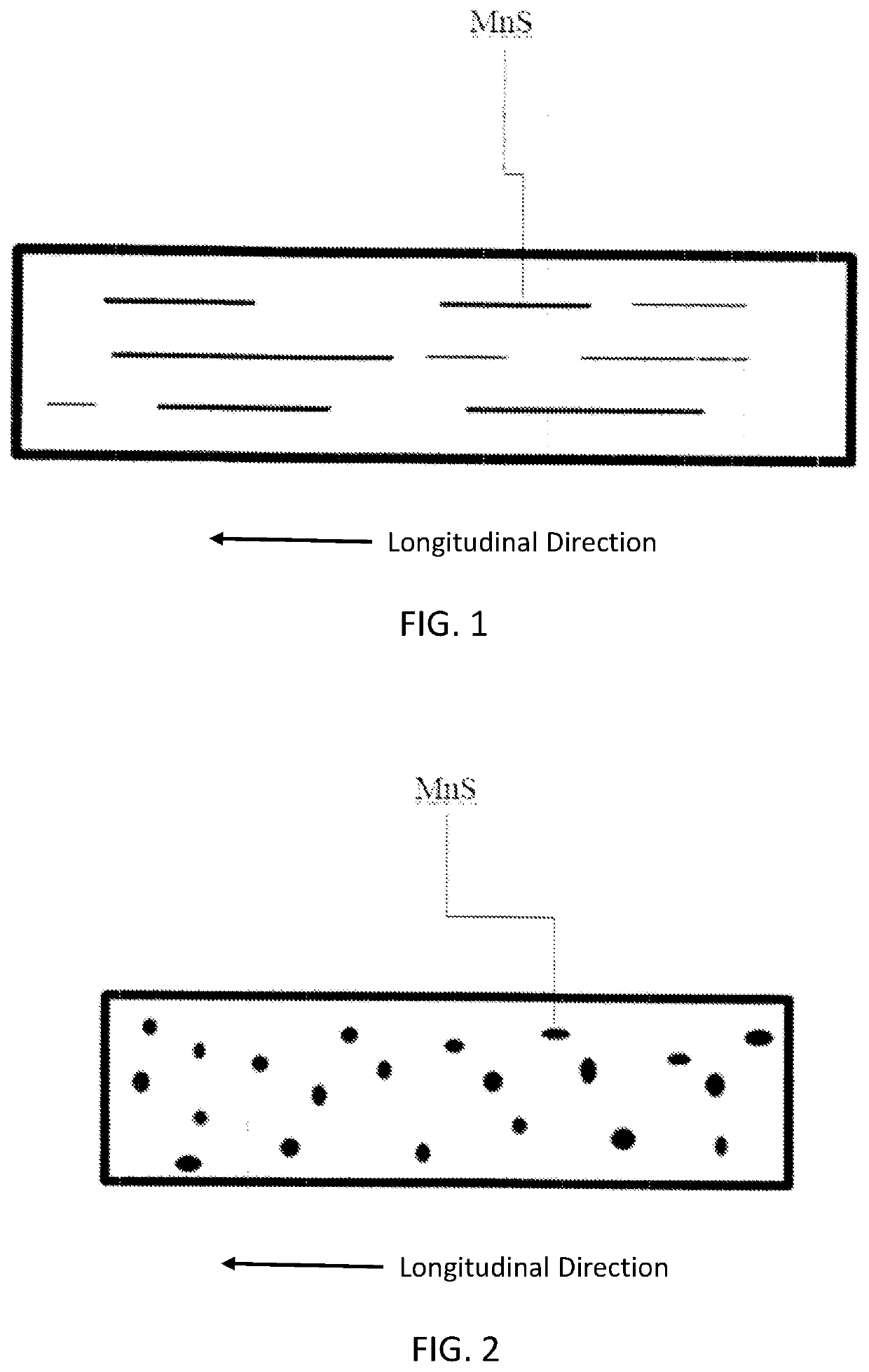
Fe-mn absorbable implant alloys with increased degradation rate
The present invention is directed to a biodegradable alloy suitable for use in a medical implant, comprising at least 50% iron by weight, at least 25% manganese by weight, and at least 0.01% sulfur and / or selenium by weight, wherein the biodegradable alloy is nonmagnetic. The present invention also provides a method of producing a biodegradable alloy with a desirable degradation rate.
Owner:BIO DG INC
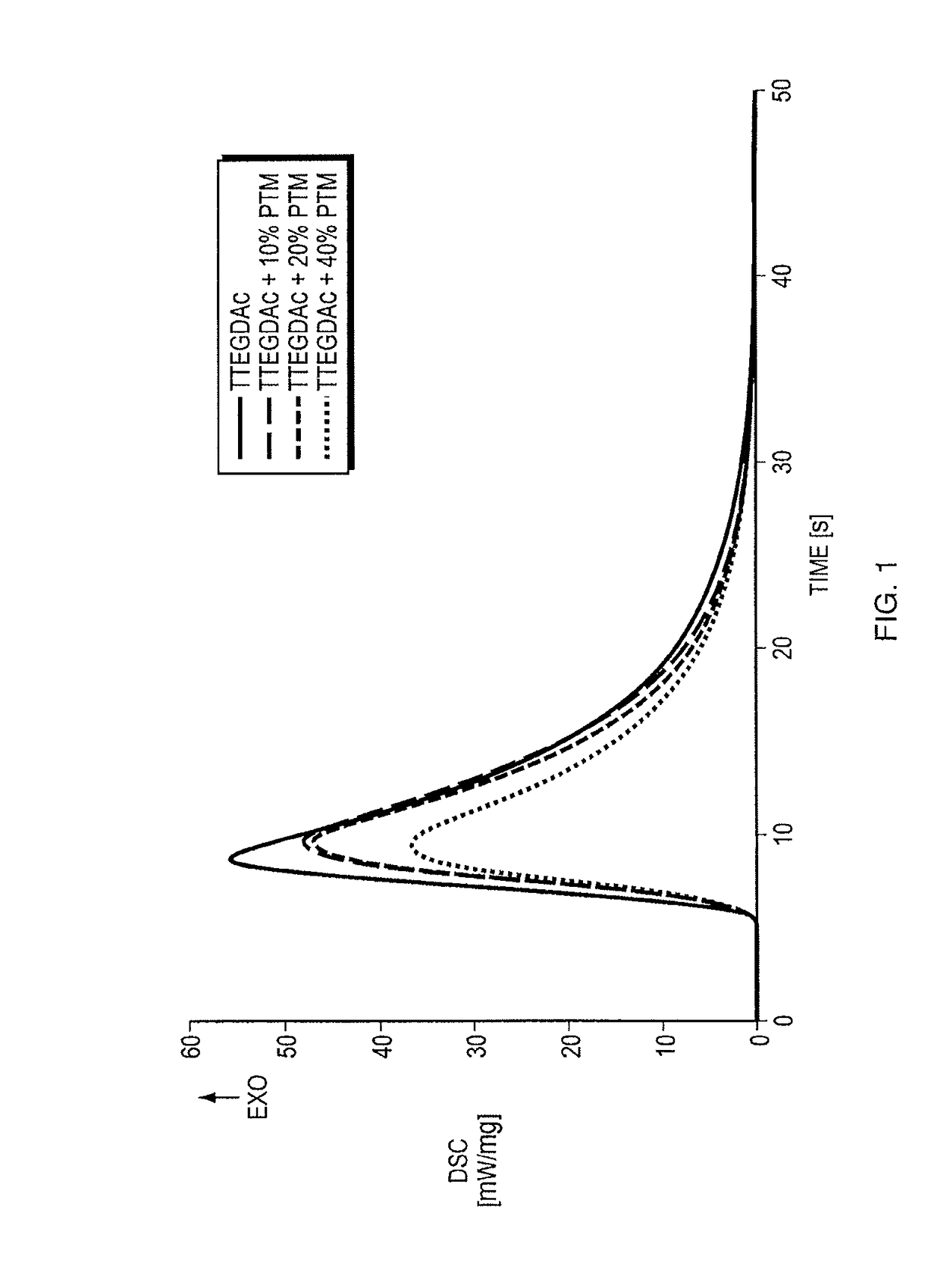
Thiol-ene polymerization with vinylesters and vinylcarbonate
ActiveUS9623145B2Improve adhesionPromote and induce bone formationElectrotherapyImpression capsThiolAbsorbable Implants
The present disclosure is directed, in part, to a curable composition, a method for augmenting a structure in a patient with a resorbable biocompatible polymer, and a biodegradable, resorbable implant comprising a biocompatible copolymer. An exemplary embodiment of the curable composition comprises (a) 60 wt. % to 95 wt. % of one or more vinyl ester monomers and / or vinylcarbonate monomers, wherein said one or more vinyl ester monomers and / or vinylcarbonate monomers are respectively selected from compounds of the general formulas (I) and (II) below:wherein n, m R1 and R2 have the meaning defined herein; (b) 0.1 to 40 wt. % of one or more multifunctional thiols; and (c) 0 to 10 wt. % of a biocompatible polymerization initiator.
Owner:DEPUY SYNTHES PROD INC +1
Iron based and absorbable implanted medical device and prefabricated tube and preparation method therefor
ActiveUS20180221536A1Small sizeImprove production efficiencyHeart valvesSurgeryNitrogenAbsorbable Implants
An iron based and absorbable implanted prefabricated tube (1) for medical device and preparation method therefor, and a medical device prepared by the prefabricated tube and preparation method therefor. The average nitrogen content of nitrogen in the prefabricated tube is from 0.04-0.4 wt. % or 0.02-0.04 wt. %. The prefabricated tube can be cut along the length direction directly by laser or by machining to obtain multiple absorbable implanted medical devices.
Owner:BIOTYX MEDICAL SHENZHEN CO LTD
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20110189304A1Lower potentialMinimally inhibit osteogenesis and subsequent bone healingOrganic active ingredientsSurgical adhesivesMedicineCarboxylic salt
The present invention relates to mechanically hemostatic body-absorbable compositions having a putty-like consistency. The compositions preferably comprise a finely powdered, carboxylic acid salt and a liquid block copolymer of ethylene oxide and propylene oxide.
Owner:ABYRX
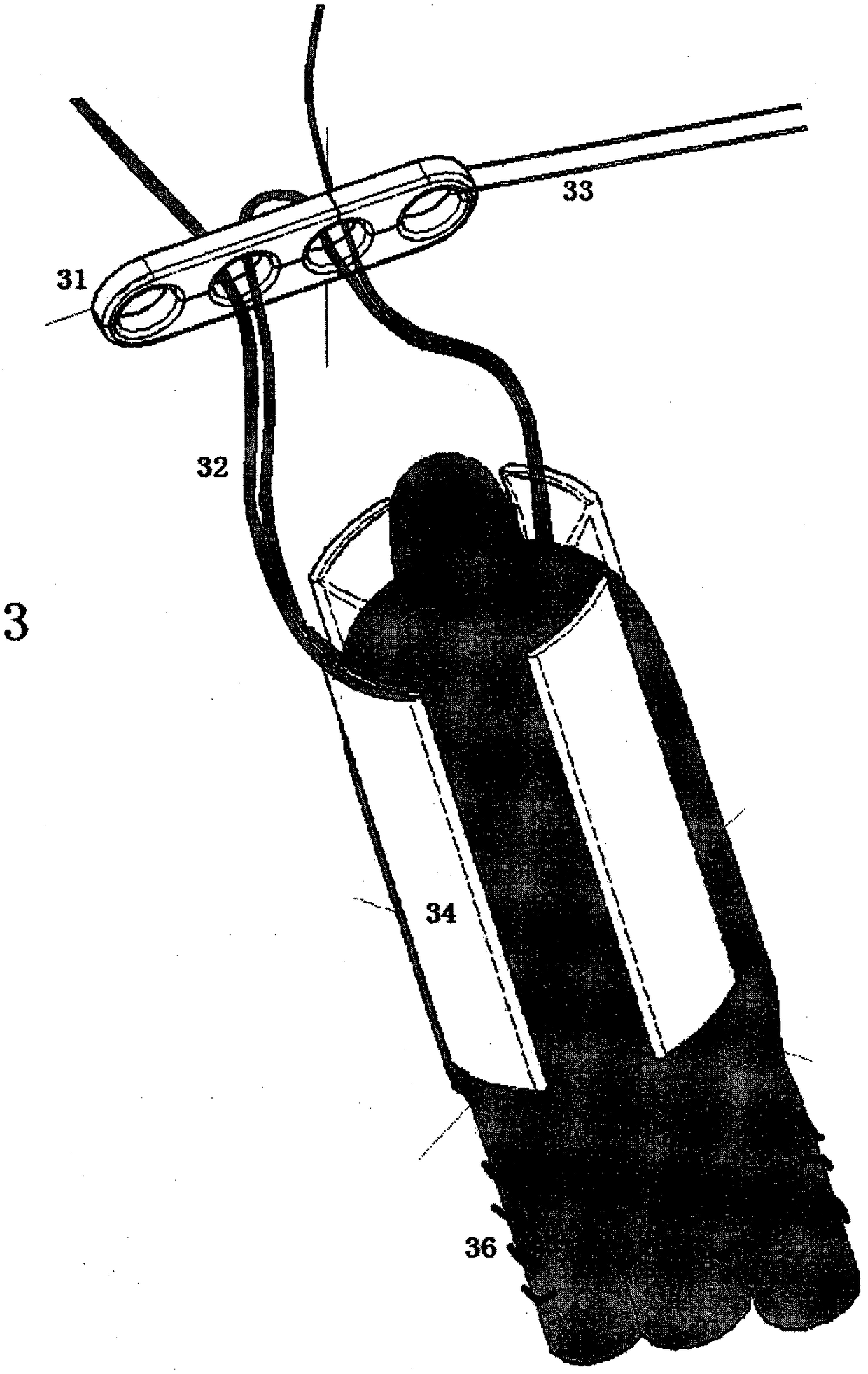
Ligament Fixation System Containing Suspension Plates and Absorbable Implants
ActiveCN108209990BReduce shear stressEasy to handleSuture equipmentsStaplesBone tunnelAbsorbable Implants
The present invention relates to a ligament fixation system comprising a suspension plate and an absorbable implant and a method of use thereof. The ligament fixation system includes a suspension plate, an absorbable implant, a suspension wire, and a suture. The implant is cylindrical, with symmetrically distributed parallel openings around it, an arc-shaped groove forming a smooth connection with the ligament graft on the upper side, and a square groove convenient for suture fixing on the lower side. The implant increases the contact area between the ligament graft and the bone tunnel surface, hinders the penetration of joint fluid into the bone tunnel, effectively reduces the "wiper effect" and prevents the frictional fracture of the ligament graft. The use of absorbable materials facilitates true tendon‑bone healing. The ligament fixation system has wide sources of raw materials, is convenient to manufacture, is quick to assemble, and is simple to use, can simplify tendon treatment steps, shorten operation time, and significantly improve the firmness of ligament grafts.
Owner:BEIJING WANJIE MEDICAL DEVICE CO LTD
Thiol-ene polymerization with vinylesters and vinylcarbonate
ActiveUS20160243279A1Improve adhesionPromote and induce bone formationOrganic active ingredientsAdditive manufacturing apparatusThiolAbsorbable Implants
The present disclosure is directed, in part, to a curable composition, a method for augmenting a structure in a patient with a resorbable biocompatible polymer, and a biodegradable, resorbable implant comprising a biocompatible copolymer. An exemplary embodiment of the curable composition comprises (a) 60 wt. % to 95 wt. % of one or more vinyl ester monomers and / or vinylcarbonate monomers, wherein said one or more vinyl ester monomers and / or vinylcarbonate monomers are respectively selected from compounds of the general formulas (I) and (II) below:wherein n, m R1 and R2 have the meaning defined herein; (b) 0.1 to 40 wt. % of one or more multifunctional thiols; and (c) 0 to 10 wt. % of a biocompatible polymerization initiator.
Owner:VIENNA UNIVERSITY OF TECHNOLOGY +1
Slow release antibiotics preparation of bioactive substance
The present invention relates to an antibiotic preparation for absorbable and non-absorbable implants used in human and veterinary medicine for the treatment of local microbial infections in hard and soft tissues. This antibiotic preparation is a mixture, which is composed of at least an amphoteric component and an antibiotic component, an anhydrous organic auxiliary component, an inorganic auxiliary component and a biologically active auxiliary component. Representatives of the amphoteric components are alkyl sulfates, aryl sulfates, alkylaryl sulfates, cycloalkyl sulfates, alkylcycloalkyl sulfates, alkyl sulfamate, cycloalkyl Sulfamate, alkylcycloalkylsulfamate, arylsulfamate, alkyl arylsulfamate, alkylsulfonate, fatty acid-2-sulfonate, arylsulfonate , Alkyl aryl sulfonate, cycloalkyl sulfonate, alkyl cycloalkyl sulfonate, alkyl pyrosulfate, cycloalkyl pyrosulfate, alkyl disulfonate, cycloalkyl disulfonate acid salt, aryl disulfonate, alkylaryl disulfonate, aryl trisulfonate and alkylaryl trisulfonate, the antibiotic component is selected from the following antibiotics: aminoglycoside antibiotics, Lincosamide antibiotics, 4-quinolone antibiotics or tetracycline antibiotics. The antibiotic preparation of the present invention can sustainably release biologically active substances.
Owner:HERAEUS KULZER
Magnesium-based absorbable implants
An implantable tissue filler including a particulate material suspended in a carrier, the particulate material having more than about 70% by weight magnesium and about 2-20% by weight lithium, wherein the particulate material is substantially free of rare earth metals.
Owner:ZORION MEDICAL
Surface modification method of bio-absorbable material implanted in polyester
The invention discloses a surface modification method of a bio-absorbable material implanted in a polyester, and the bio-absorbable material implanted in the polyester is treated by supercritical fluid. The invention utilizes the supercritical carbon dioxide technology to modify the surface of various existing internally absorbable implanted materials, so as to form holes with nanometer size or micron size on the surface of the material, the invention solves the problem that the interface of existing internally absorbable implanted material is not suitable for cell adhesion and proliferation, thereby facilitating protein adsorption and deposition of extracellular matrix, and integration with the surrounding tissues; moreover, the invention can form tiny blood vessels timely, and can achieve good synchronization of material degradation and replacement of bone cells.
Owner:CHANGCHUN SINOBIOMATERIALS
Absorbable implant material composed of magnesium or magnesium alloy containing doped nanodiamonds
The present invention relates to an absorbable implant material composed of magnesium or a magnesium alloy and to a method for the production thereof. A disadvantage of the known absorbable implants is that the position of the implant during the medical implantation procedure and immediately thereafter can only be tracked by means of X-ray examinations. According to the invention, the absorbable implant material comprising homogeneously distributed Fe-doped nanodiamonds in a matrix composed of magnesium or the magnesium alloy, is provided. The Fe-doped nanodiamonds are harmless to organisms soas to allow the detection of the implant material in the blood plasma of the patient by means of magnetic resonance imaging. According to the invention, the absorbable implant material according to the invention is produced by a method in which the magnesium or the magnesium alloy is melted, the Fe-doped nanodiamonds are added to a melt, and the melt composed of the magnesium or the magnesium alloy that has been provided with Fe-doped nanodiamonds is subjected to an ultrasound treatment.
Owner:HELMHOLTZ ZENT GEESTHACHT ZENT FUER MATERIAL UND KUESTENFORSCHUNG
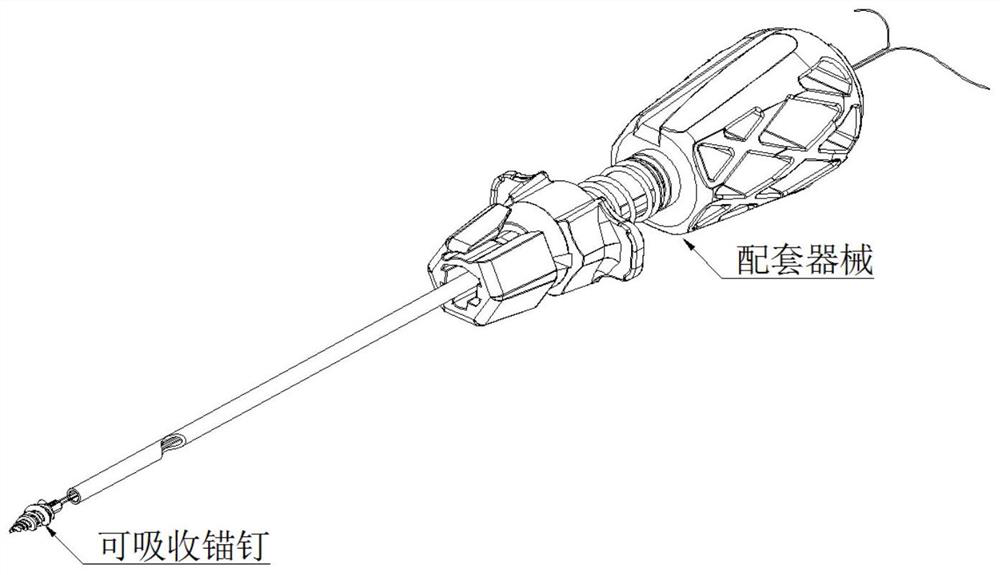
Sterilization method for absorbable implant and matched instrument combined product
ActiveCN112999388AAchieve sterile effectReduce performanceLavatory sanitoryCleaning using liquidsEthylene Oxide SterilizationAbsorbable Implants
The invention belongs to the field of medical instrument sterilization, and relates to a sterilization method for an absorbable implant and matched instrument combined product. The method comprises the following steps: cleaning non-absorbable instrument sub-components in a clean room, assembling into a non-absorbable matched instrument, carrying out ethylene oxide sterilization or radiation sterilization on the non-absorbable matched instrument, assembling the absorbable implant consumables on the sterilized non-absorbable matched instrument in a clean room, and then performing ethylene oxide sterilization and desorption. By adopting the method provided by the invention, the contradictions that the absorbable implant consumables in the absorbable implant and the matched instrument combined product cannot be subjected to excessive sterilization due to the characteristic of easy degradation and the sterilization effect cannot be ensured due to the complex structure of the non-absorbable matched instrument can be effectively solved; and the method can guarantee that the absorbable implant consumables and matched instruments with complex structures can achieve the sterile effect at the same time, the performance of the absorbable implant consumables cannot be reduced, and important practical use value and significance are achieved.
Owner:厦门博创中安医疗技术有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com