Surface modification method of bio-absorbable material implanted in polyester
An implant material and surface modification technology, used in prosthesis, medical science, surgery, etc., can solve the problems of small number of holes, difficult to achieve tissue fluid, nutrient transport and exchange, unfavorable cell adhesion and proliferation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Put the polylactic acid (PLA) absorbable vertebral body fusion device in the autoclave;
[0039] 2. Put the reaction kettle into the oil bath and adjust the temperature to 55°C;
[0040] 3. Open CO 2 Cylinder gas valve, open the supercritical fluid pump, and the reactor inlet valve, close the reactor vent valve; pass CO into the reactor 2 , and repeated ventilation several times;
[0041] 4. Adjust the supercritical fluid pump output CO 2 Pressure constant pressure to 20MPa;
[0042] 5. Keep the reactor at a constant temperature of 55°C and a constant pressure of 20MPa for 1 hour;
[0043] 6. After the reaction is over, depressurize and remove CO 2 , the control time is 10 minutes.
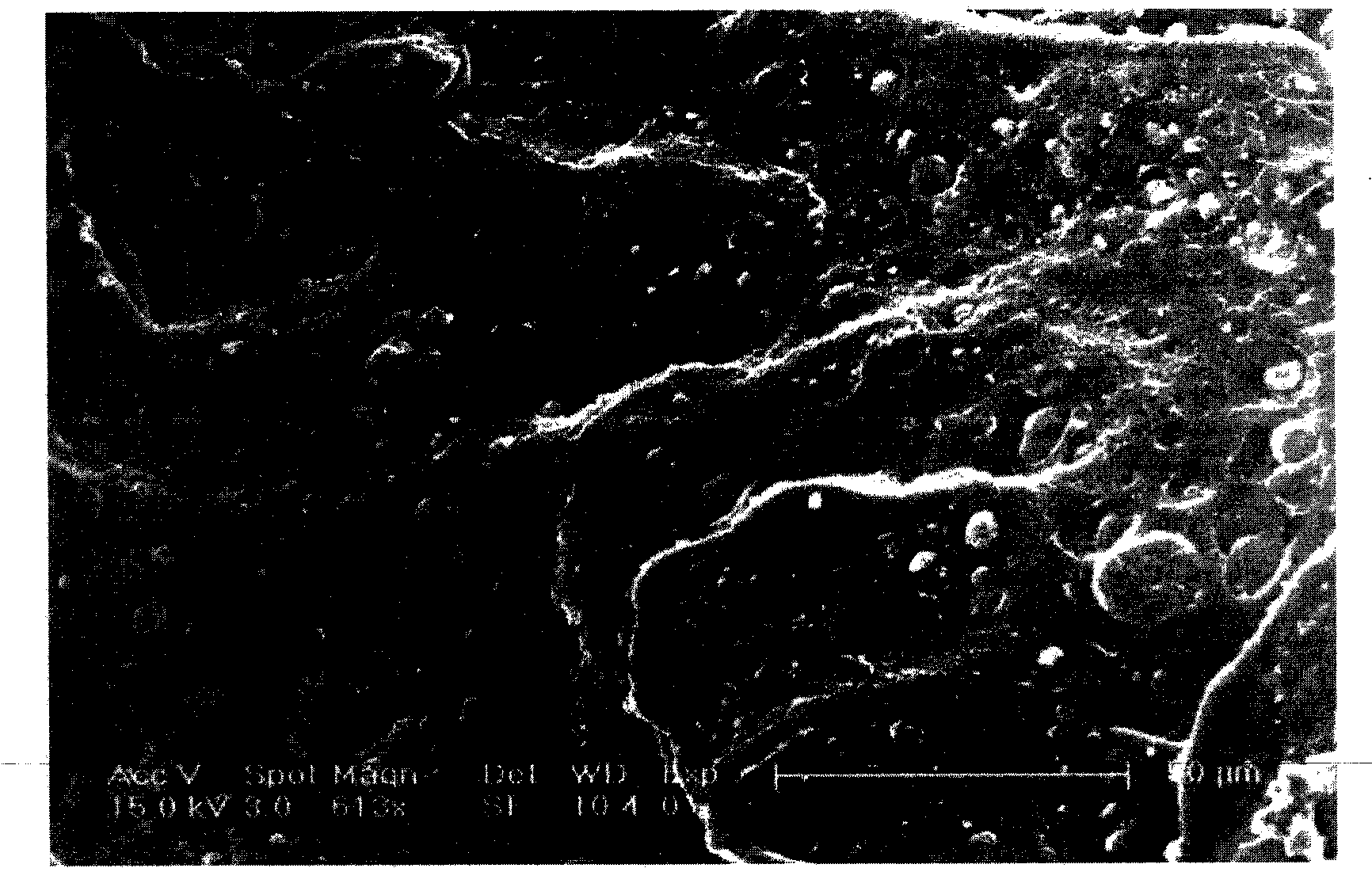
[0044] 7. Take out the surface-modified absorbable vertebral fusion cage, dry it in vacuum, and detect the surface changes by field emission scanning electron microscopy (ESEM). Such as figure 1 shown.
Embodiment 2
[0046] 1. Put polylactic acid (10% HA / PLA) grafted 10% hydroxyapatite absorbable thoracic fusion device in an autoclave;
[0047] 2. Put the reaction kettle into the oil bath and adjust the temperature to 55°C;
[0048] 3. Open CO 2 Cylinder gas valve, open the supercritical fluid pump, and the reactor inlet valve, close the reactor vent valve; pass CO into the reactor 2 , and repeated ventilation several times;
[0049] 4. Adjust the supercritical fluid pump output CO 2 Pressure constant pressure to 20MPa;
[0050] 5. Keep the reactor at a constant temperature of 55°C and a constant pressure of 20MPa for 1 hour;
[0051] 6. After the reaction is over, depressurize and remove CO 2 , the control time is 10 minutes.
[0052] 7. The surface-modified absorbable thoracic fusion cage was taken out, dried in vacuum, and the surface changes were detected by a field emission scanning electron microscope (ESEM). Such as figure 2 shown.
Embodiment 3
[0054] 1. Put polylactide glycolide (PLGA) in vivo absorbable vascular stent into the autoclave;
[0055] 2. Put the reaction kettle into the oil bath and adjust the temperature to 45°C;
[0056] 3. Open CO 2 Cylinder gas valve, open the supercritical fluid pump, and the reactor inlet valve, close the reactor vent valve; pass CO into the reactor 2 , and repeated ventilation several times;
[0057] 4. Adjust the supercritical fluid pump output CO 2 Pressure constant pressure to 20MPa;
[0058] 5. Keep the reactor at a constant temperature of 45°C and a constant pressure of 20MPa for 3 hours;
[0059]6. After the reaction is over, depressurize and remove CO 2 , the control time is 10 minutes.
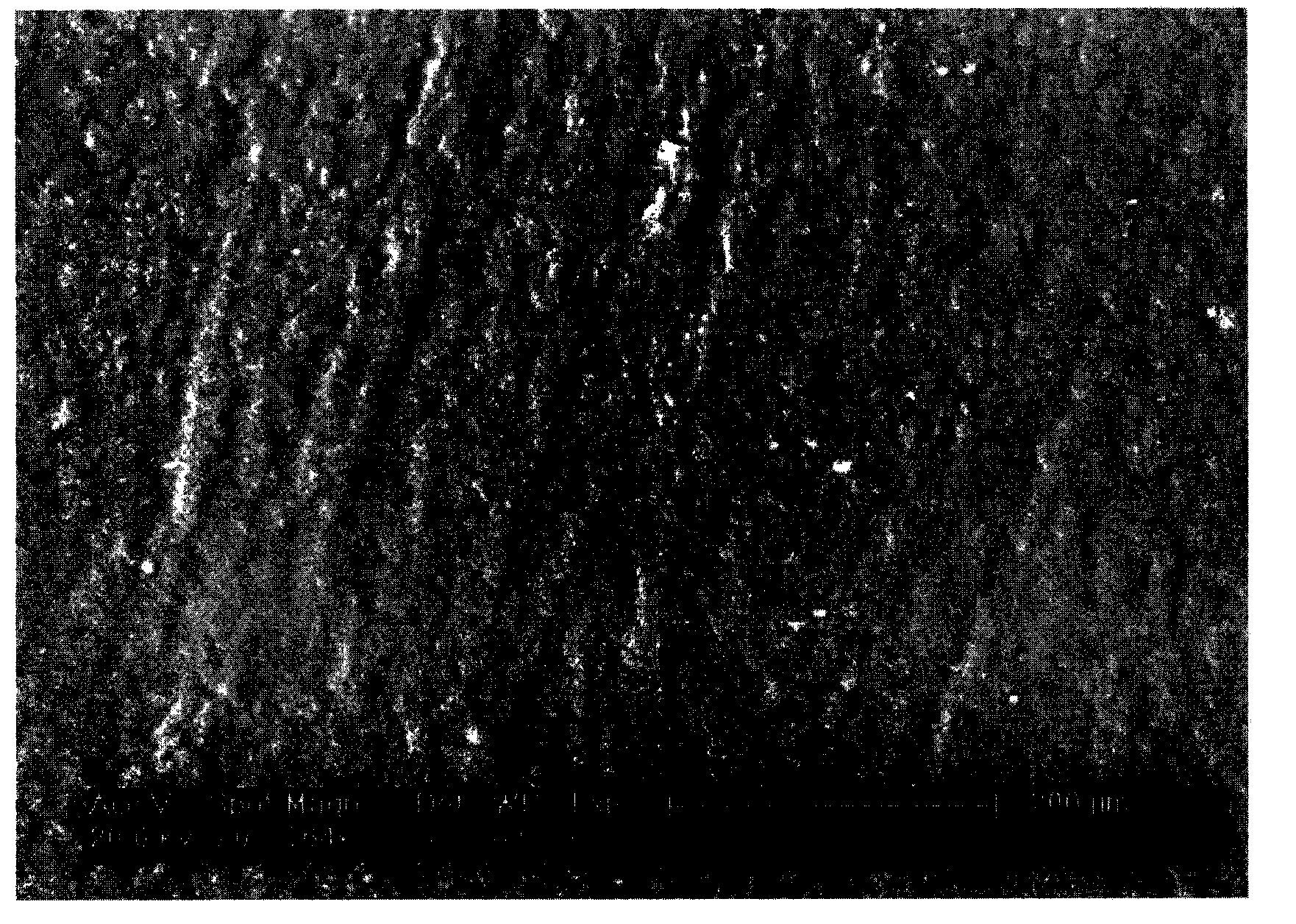
[0060] 7. Take out the surface-modified absorbable vascular stent, dry it in vacuum, and detect the surface change by a field emission scanning electron microscope (ESEM). Such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com