Pharmaceutical composition comprising ginsenosides Rh3, PPD and Rh2
A technology of ginsenosides and compositions, which is applied in the field of rare ginsenosides Rh3/PPD/Rh2 compositions, and can solve problems such as anti-tumor effects that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
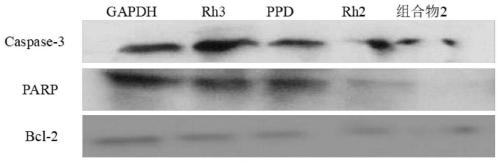
[0079] Example 1 Ginsenoside Rh3, ginsenoside PPD, ginsenoside Rh2, and ginsenoside Rh3 / PPD / Rh2 compositions, Rh3 / PPD compositions, PPD / Rh2 compositions, and Rh3 / Rh2 compositions have effects on gastric cancer cells, liver cancer cells, Inhibition of pancreatic cancer cells
[0080] The aqueous solutions of Rh3, PPD and Rh2 with a concentration of 150ug / mL were prepared respectively. According to the weight ratio of ginsenoside Rh3:PPD:Rh2 respectively 1:1:1, 1:1:2, 1:2:1, 1:0.5:0.5, 1:3:3 to prepare the total concentration of 150ug / mL containing Rh3 , PPD, the aqueous solution of Rh2, it is referred to as composition 1, composition 2, composition 3, composition 4, composition 5; Press ginsenoside Rh3:PPD, PPD:Rh2, Rh3:Rh2 weight ratio is 1 respectively :1:, 1:2, 1:2 to prepare the aqueous solution containing Rh3, PPD with a total concentration of 150ug / mL; PPD:Rh2, Rh3:Rh2, which are referred to as composition 6, composition 7, and composition 8; Ginsenoside Rh3:PPD, PPD:Rh...
Embodiment 2
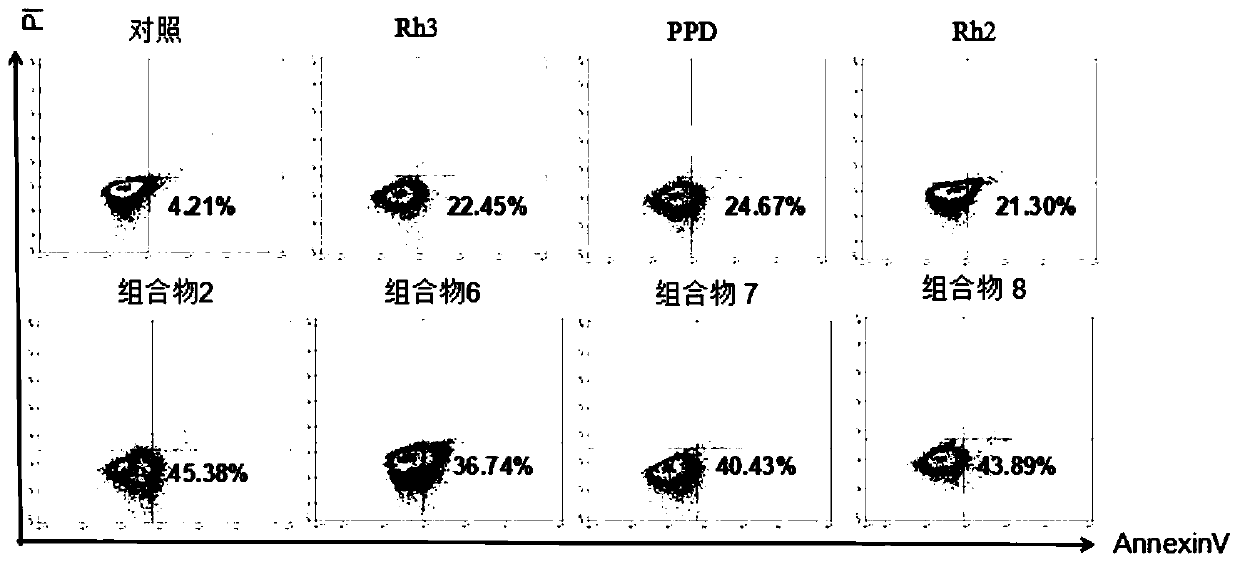
[0097] Example 2 Effects of Ginsenoside Rh3, Ginsenoside PPD, Ginsenoside Rh2, and Ginsenoside Rh3 / PPD / Rh2 Composition 2, Composition 6, Composition 7, and Composition 8 on Inducing Gastric Cancer Cell Apoptosis
[0098] Taking gastric cancer cells as an example, the cells were inoculated in a sterile 6-well plate, and after 24 hours of culture, 150ug / mL ginsenoside Rh3; 150ug / mL ginsenoside PPD; 150ug / mL ginsenoside Rh2 and 150ug / mL Ginsenoside composition, ginsenoside composition is ginsenoside composition 2, composition 6, composition 7, composition 8 in embodiment 1. An equal volume of 1640 culture solution was added to the control group, and 5 replicate wells were set for each concentration. After culturing for 24 hours, add ice-cold PBS to wash 2-3 times, add 1×Binding Buffer buffer, pipette evenly, then drop an appropriate amount of AV / PI mixed dye solution, incubate for 15 minutes in the dark, and use flow cytometry to detect cell apoptosis Death.
[0099] figure 1 ...
Embodiment 3
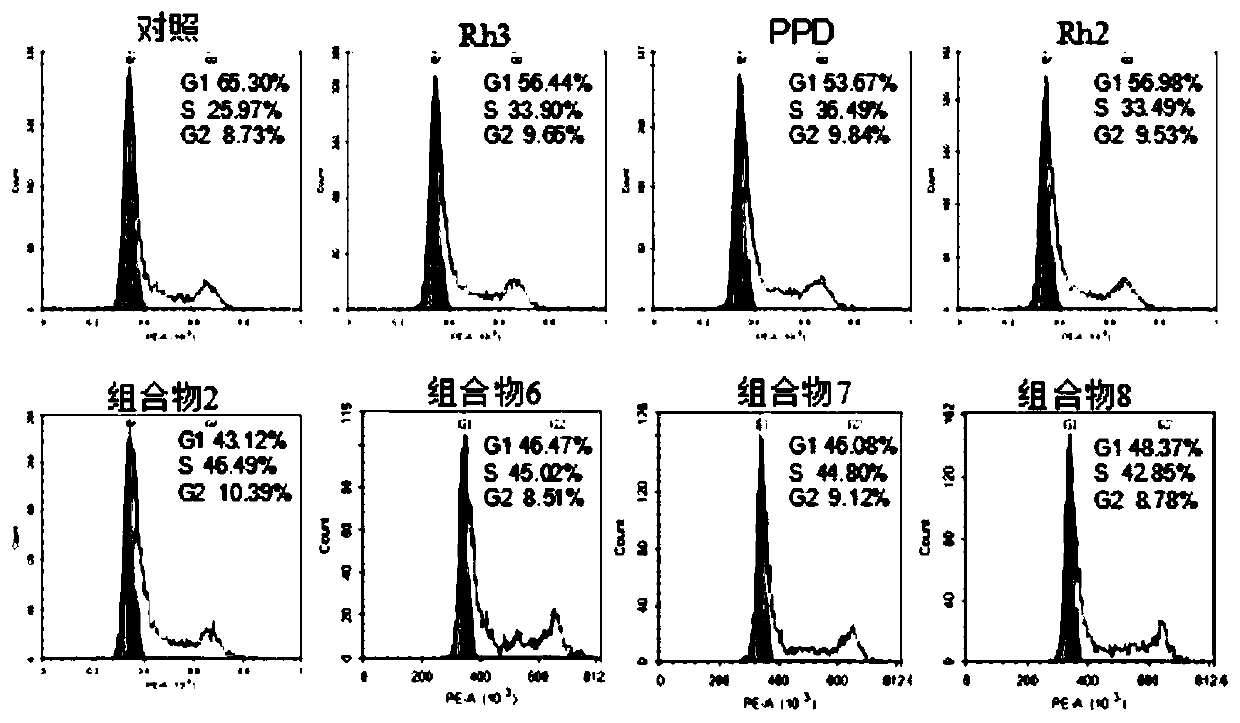
[0104] Example 3 Effects of ginsenoside Rh3, ginsenoside PPD, ginsenoside Rh2, and ginsenoside Rh3 / PPD / Rh2 compositions 2, 6, 7, and 8 on the cycle distribution of gastric cancer cells
[0105] Taking gastric cancer cells as an example, the cells in the logarithmic growth phase were divided into 1×10 5 Inoculate each well in a 6-well plate, add ginsenoside Rh3, ginsenoside PPD, ginsenoside Rh2, and composition 2, composition 6, composition 7, and composition 8 at a final concentration of 150 μg / mL, and add and add drugs to the control group Set an equal volume of RPMI-1640 culture solution, and set 5 duplicate wells for each concentration. After continuing to act for 48 hours, the cells were digested and centrifuged, the supernatant was removed, washed 2-3 times by adding 4°C pre-cooled PBS, then added 4°C pre-cooled 75% ethanol, and fixed overnight at 4°C. The next day, discard the supernatant by centrifugation, then add 4°C pre-cooled PBS to wash 2-3 times, add an appropria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com