Methods and compositions for genetically modifying and expanding lymphocytes and regulating the activity thereof
A technology of lymphoid tissue and cells, applied in the field of immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
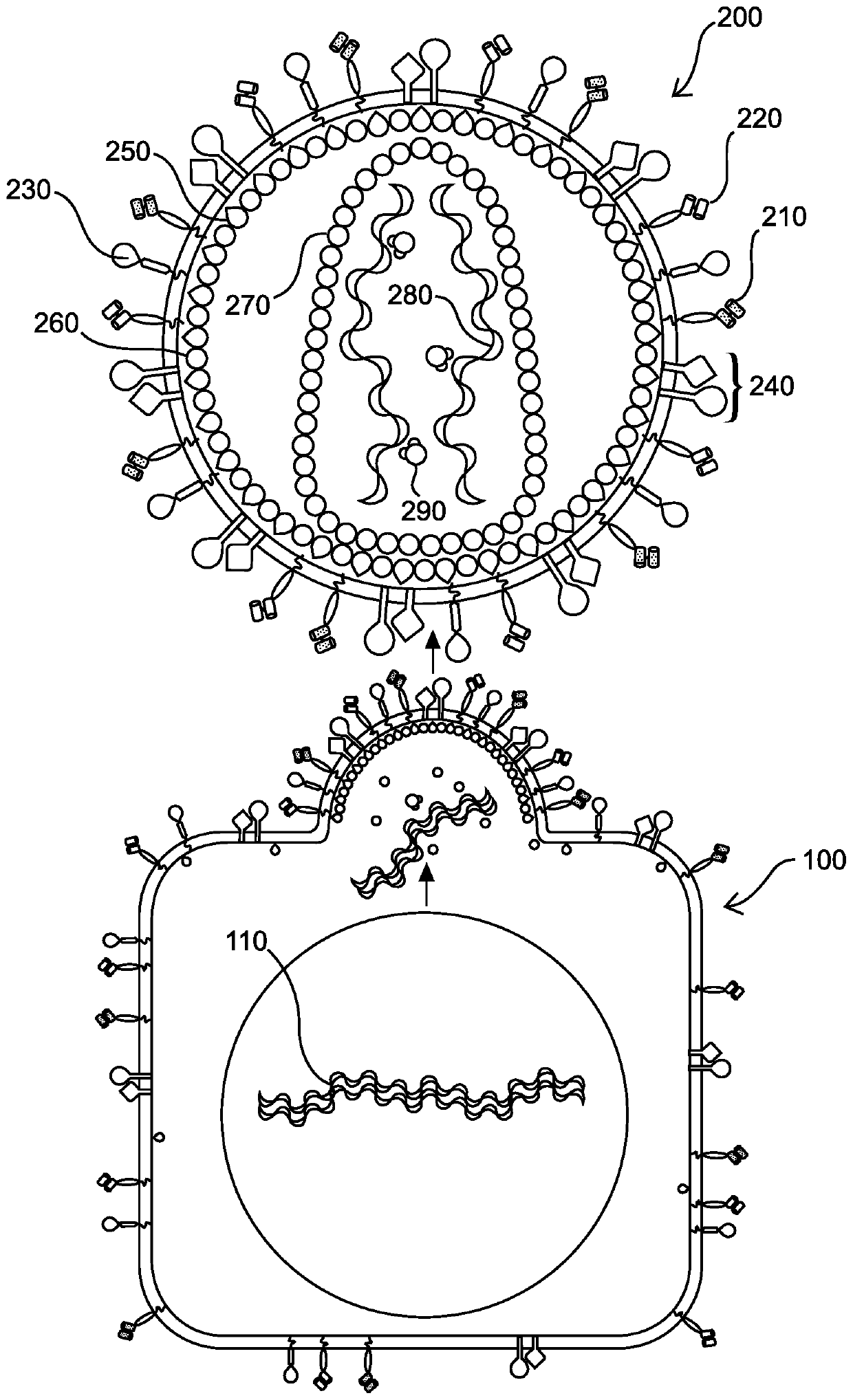
[0129] In certain aspects, provided herein are methods for performing adoptive cell therapy on an individual, as an illustrative example, the methods may include the following:
[0130] A. Collecting blood from an individual;
[0131] B. Isolation of peripheral blood mononuclear cells (PBMC) comprising resting T cells and / or resting NK cells;
[0132]C. Ex vivo contacting the individual's resting T cells and / or resting NK cells with a replication-defective recombinant retroviral particle comprising on its surface a protein capable of binding to resting T cells and / or NK cells and pseudotyped components that facilitate fusion of the replication-defective recombinant retroviral particle with its membrane, wherein the contacts facilitate transduction of quiescent cells with the replication-defective recombinant retroviral particle T cells and / or NK cells, thereby generating genetically modified T cells and / or NK cells; and
[0133] D. Reintroducing the genetically modified cell...
example 1
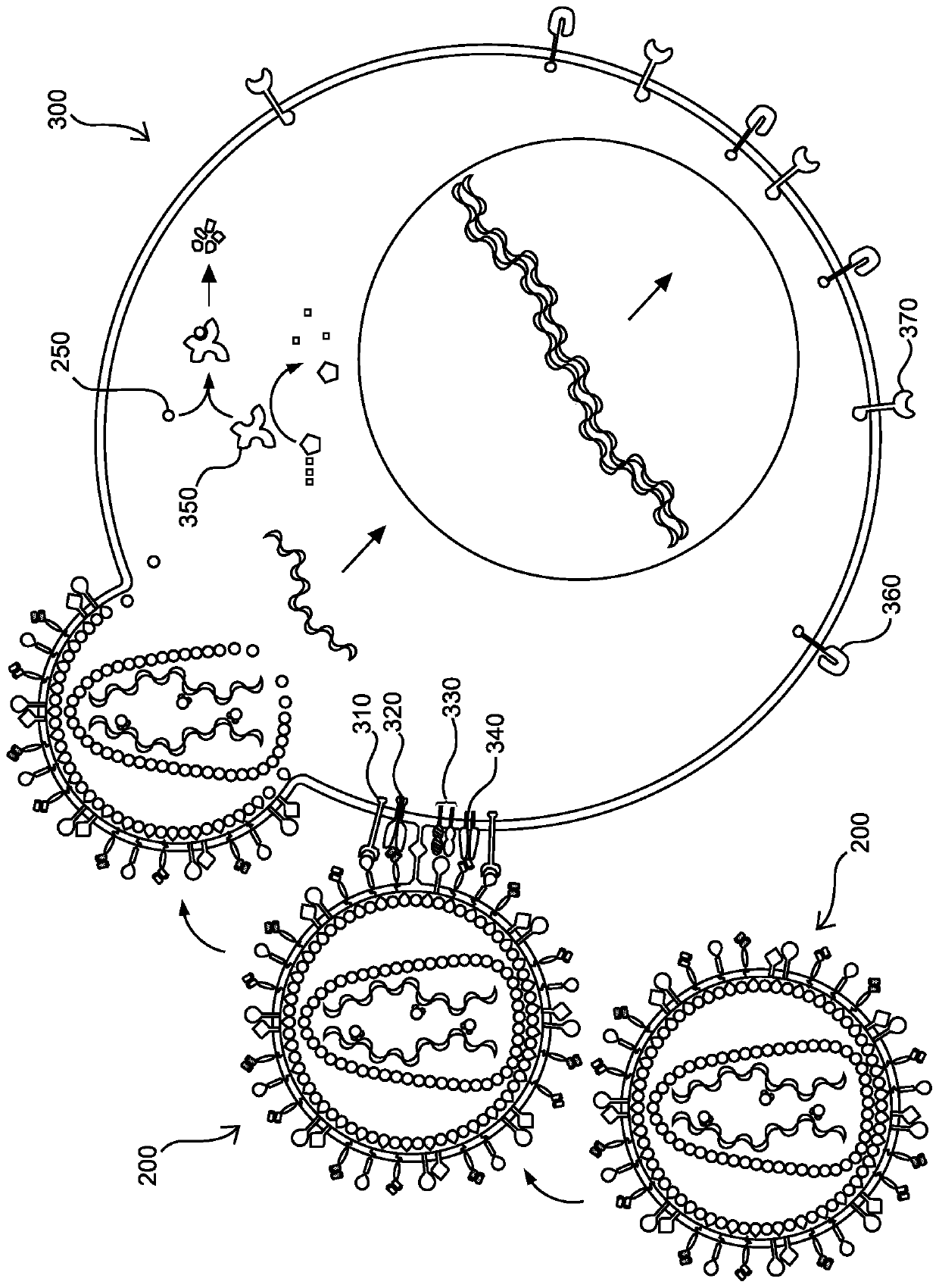
[1026] Example 1. Engineering of a retroviral packaging and transduction system to target resting T cells for selection of T cell integration and expression by PBMCs.
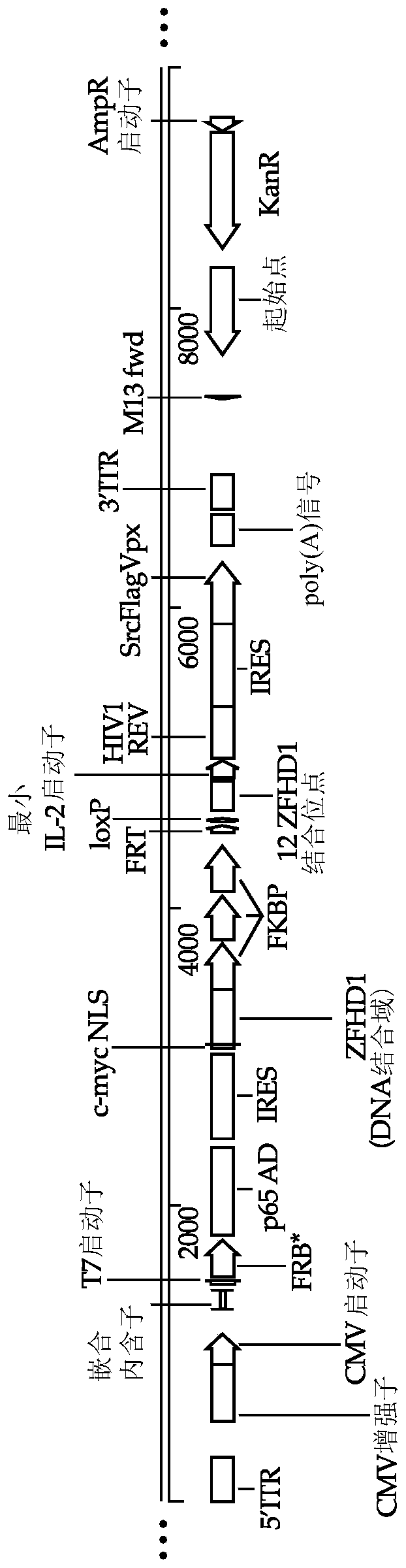
[1027] Although it is possible to generate high-titer lentiviral vectors using transient transfection, this method carries the risk of producing replication-deficient recombinant retroviruses (RCRs) and is not scalable for clinical applications. Here, a stable CAR gene was generated by synchronously introducing multiple constructs encoding inducible promoters and their regulators into HEK293 suspension-adapted cells (HEK293S) to stably produce viral components, CAR genes and their regulatory components. Retroviral packaging cell lines. Two different inducible systems can be used to temporarily control gene expression. One system is based on rapamycin- or rapamycin analog-induced dimerization of two transcription factors. One transcription factor consists of three copies of the FKPB protein fused to the ZFHD1 ...
example 2
[1031] Example 2. Production of lentiviral vectors and retroviral packaging.
[1032] With the construct for expressing Flp recombinase ( Figure 4C ) and the retroviral packaging stable cell line produced in Example 1 transfected with a construct containing a polynucleotide sequence encoding a CAR and under the control of the CD3Z promoter (which is inactive in HEK293S cells) The lymphoproliferative component IL7Rα-insPPCL, wherein CAR and IL7Rα-insPPCL are separated by a polynucleotide sequence encoding T2A ribosomal skipping sequence, and IL7Rα-insPPCL has ribonuclease controlled by acyclovir riboswitch. The CAR-containing construct further includes cPPT / CTS and RRE sequences and polynucleotide sequences encoding HIV-1 Psi. The entire polynucleotide sequence on the CAR-containing construct to be integrated into the genome is flanked by FRT sites. Successful integration of the CAR-containing construct resulted in constitutive expression of GFP that was thus removed by tran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com