New use of compound ZL0580 for preventing or treating African swine fever
A technology of African swine fever and African swine fever virus, which is applied in the field of African swine fever treatment, can solve problems such as economic loss and inability to meet large-scale pig raising, and achieve the effect of enhancing immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
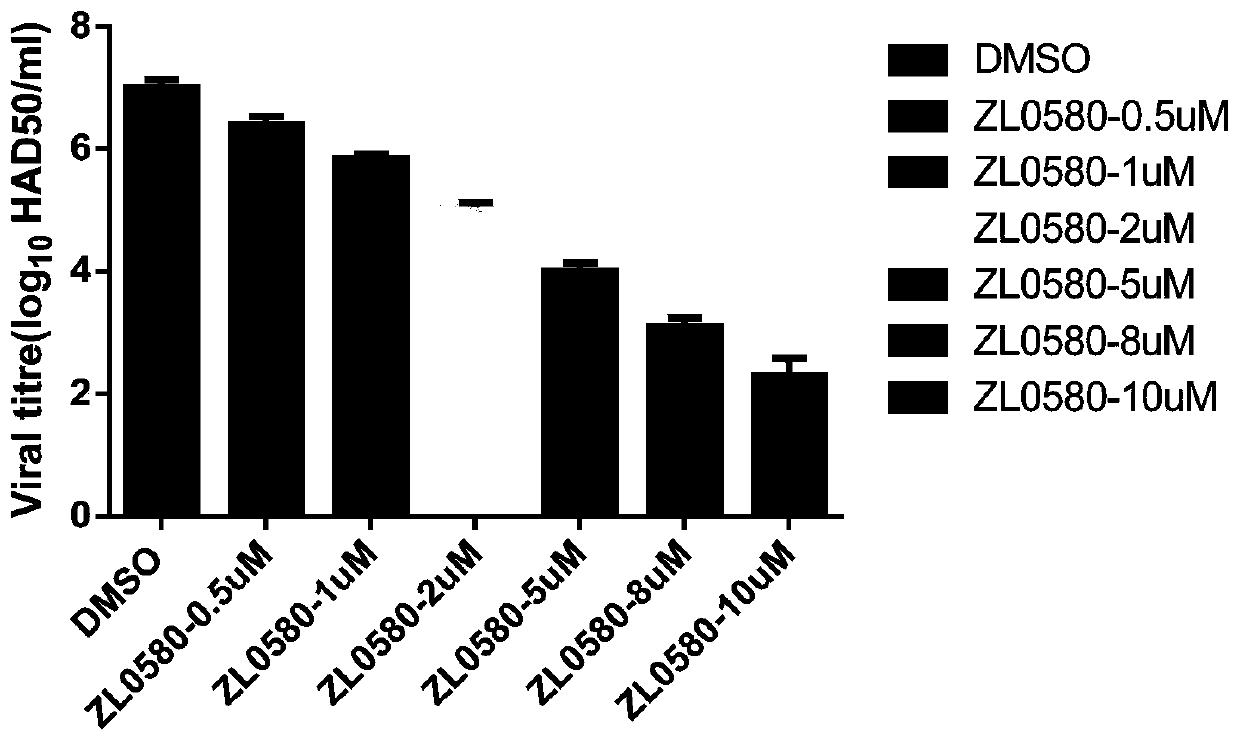
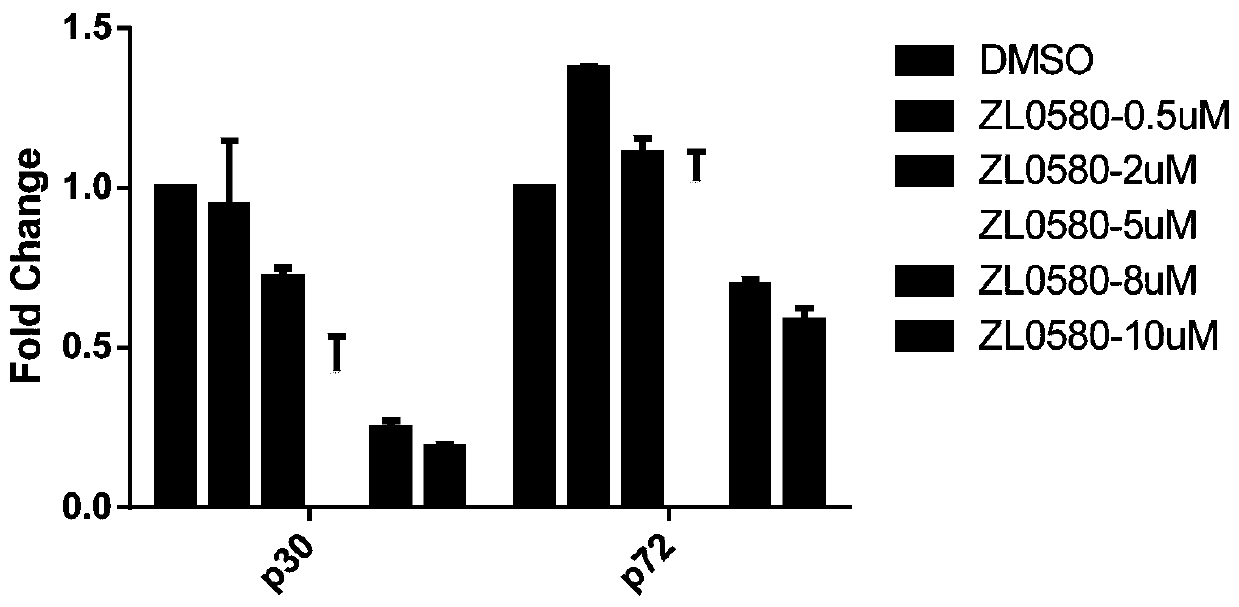
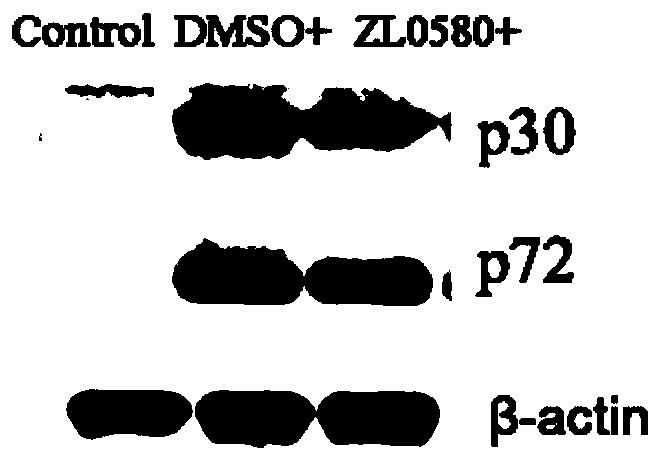
[0038] Example 1 The effect of compound ZL0580 on the replication of African swine fever virus infection and the transcription and expression of genes
[0039] 1. Changes in African swine fever virus infection and replication
[0040] Porcine alveolar macrophages (PAM, 2 × 10 5 / well), the experimental group treated the cells with different concentrations of ZL0580 (0.5 μM, 1 μM, 2 μM, 5 μM, 8 μM, 10 μM) for 16 hours, and the infection control group treated the cells with DMSO (1%) for 16 hours; serially diluted 10 times with PBS ASFV CN / SC / 2019 strain (MOI=0.1), make 8 dilutions, repeat 8 wells for each dilution, inoculate PAM cells for culture, and add porcine red cells at the same time; place the cell plate at 37°C, 5%CO 2 The conditions were cultured for 3-6 days, and the hematocyte adsorption reaction (HAD) in each cell culture well was observed every day.
[0041] The hematocyte adsorption reaction (HAD) is based on the phenomenon that porcine erythrocytes will adsorb ...
Embodiment 2
[0052] Example 2 Effect of Compound ZL0580 on Expression Levels of Host Inflammation-Related Factors
[0053] Porcine alveolar macrophages (PAM, 1 × 10 6 / well); the experimental group was treated with ZL0580 (10 μM) for 16 hours, and then infected with ASFV CN / SC / 2019 strain (MOI=0.1); the blank control group was not treated; the compound ZL0580 treatment group was treated with ZL0580 (10 μM) The cells were treated with DMSO (1%) for 16 hours in the virus alone infection group, and then directly infected with the ASFV CN / SC / 2019 strain (MOI=0.1); the uninfected control group was treated with DMSO (1%) for 16 hours; After the above separately treated cells were cultured for 48 hours, the cell culture was collected, the cells were washed once with PBS, centrifuged, and the supernatant was discarded. Total RNA was extracted, and after cDNA was reverse transcribed, the difference in expression of host inflammation-related factors was detected by Q-PCR method (same as above).
...
Embodiment 3
[0059] The cytotoxicity of embodiment 3 compound ZL0580
[0060] By constructing a stable in vitro cell screening system, the cytotoxicity of the small molecule compound ARV-825 was detected by the CCK-8 method. Porcine alveolar macrophages (PAM, 2 × 10 5 Cultivate overnight, add different concentrations of ZL0580 (0.5 μM, 1 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM, 160 μM, 240 μM) to the wells, set blank wells (only containing medium), and control wells ( containing cells and medium), incubate the culture plate in the incubator for 16 hours, add 10 μL of CCK-8 solution to each well of the plate, incubate the culture plate in the incubator for 1-4 hours, read the plate Beforehand, mix gently on a shaker. Then read the microplate reader to measure the absorbance at 450nm, and calculate the cell viability.
[0061] The result is as Figure 5 As shown, the compound ZL0580 has little toxicity to cells, and even when the dose reaches 20 μM, the cell activity can still reach more th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com