Azacyclic carbene carboxylate bidentate ligand, bidentate ruthenium complex and preparation method and application of catalyzing carboxylic acid-alkyne addition
A technology for carbene carboxylic acid and ruthenium complexes, which is applied in the field of carbene ruthenium complex synthesis, can solve the problems of poor stability of metal ruthenium catalysts, harsh reaction conditions, short service life, etc., and achieves good catalytic activity, high catalytic yield, and durability. saved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
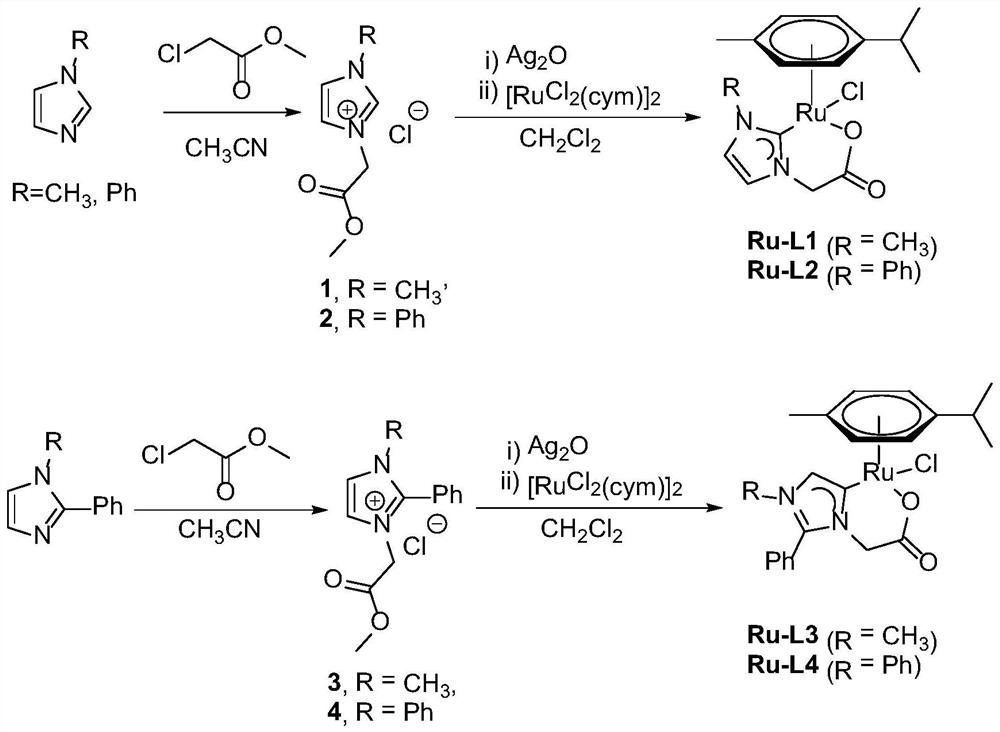
[0037] Embodiment 1: the synthesis of Ru-L1, Ru-L2, synthetic route is as figure 1 shown.
[0038] 1. Synthesis of nitrogen heterocyclic carbene carboxylate bidentate ligand L1: 5 mmol of 1-methylimidazole and 5.5 mmol of methyl chloroacetate were reacted in 7 mL of acetonitrile at 90° C. for 24 hours, then the solvent was drained, and diethyl ether was used to Get washed.
[0039] Synthesis of nitrogen heterocyclic carbene carboxylate bidentate ligand L2: 5 mmol of 1-phenylimidazole and 5.5 mmol of methyl chloroacetate in 7 mL of acetonitrile, reacted at 90 ° C for 24 hours, dried the solvent, and washed with ether to obtain .
[0040]
[0041] 2. Synthesis of Ru-L1 and Ru-L2
[0042]
[0043] Mix the azacyclic carbene carboxylate bidentate ligand L1 / azacyclic carbene carboxylic acid bidentate ligand L2 (0.5 mmol) and silver oxide (0.66 mmol) in 10 mL of dichloromethane, and react in the dark at room temperature for 24 Hours, the filtered silver carbene intermediate...
Embodiment 2
[0046] Embodiment 2: the synthesis of Ru-L3, Ru-L4, synthetic route is as figure 1 shown.
[0047] 1. Synthesis of nitrogen heterocyclic carbene carboxylate bidentate ligand L3: 5 mmol of 1-methyl-2-phenylimidazole and 5.5 mmol of methyl chloroacetate in 10 mL of acetonitrile, reacted at 90 ° C for 24 hours The solvent was drained and washed with ether.
[0048] Synthesis of nitrogen heterocyclic carbene carboxylate bidentate ligand L4: 5mmol 1,2-diphenylimidazole and 5.5mmol methyl chloroacetate were reacted in 10mL acetonitrile at 90°C for 24 hours, then the solvent was drained, and the obtained by washing with ether.
[0049]
[0050] 2. Synthesis of Ru-L3 and Ru-L4
[0051]
[0052] Mix the azacyclic carbene carboxylate bidentate ligand L3 / azacyclic carbene carboxylic acid bidentate ligand L4 (0.5mmol) and silver oxide (0.66mmol) in 10mL of dichloromethane, and react in the dark at room temperature for 24 Hours, the filtered silver carbene intermediate solution w...
Embodiment 3- Embodiment 11
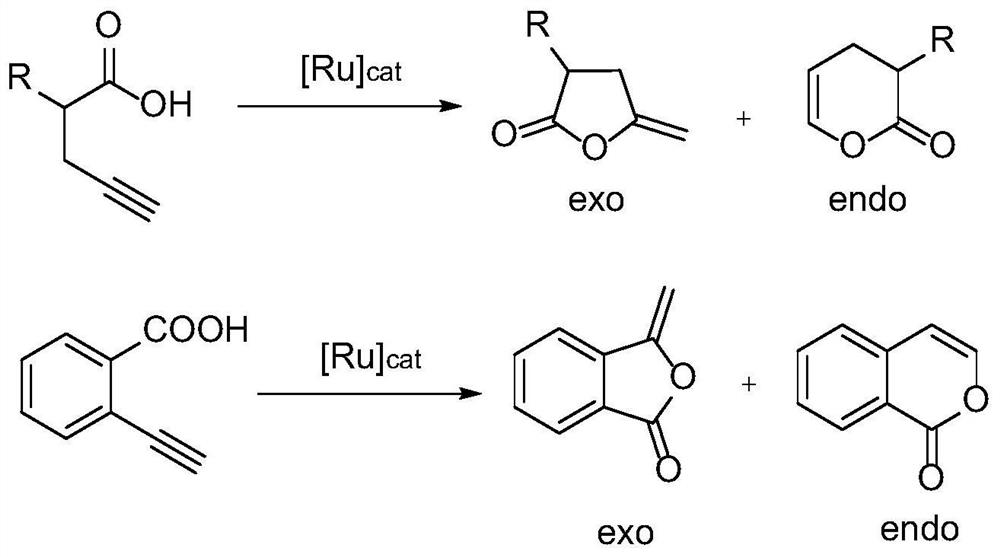
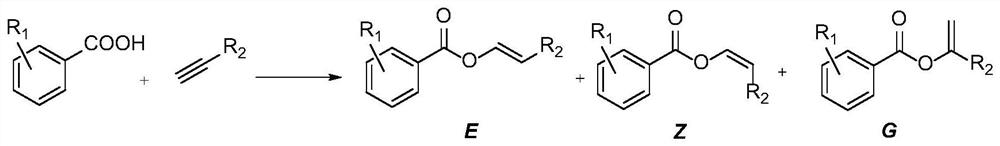
[0056] Embodiment 3-Example 11: Intramolecular addition reaction of alkynoic acid in deuterated chloroform, synthetic route such as figure 2 shown.
[0057] With 0.2mmol alkynoic acid as the reaction substrate, dissolve in 1mL CDCl 3 In, add the nitrogen heterocyclic carbene carboxylate bidentate ruthenium complex of different substance amounts (catalyst amount is the percentage of the amount of alkynoic acid substance), the sealing reaction temperature is 60 ℃, examines reaction time respectively 4h, 2 / 3h and 24h The reaction situation, after the reaction is completed, it is directly transferred to the NMR tube, and the NMR spectrum of the crude product is tested, and the NMR yield is calculated accordingly.
[0058] The catalyzer of embodiment 3-embodiment 11, catalyst addition, reaction time namely productive rate are as shown in table 1.
[0059] Table 1 shows the catalytic condition screening and substrate expansion of carboxylic acid-alkyne intramolecular addition rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com