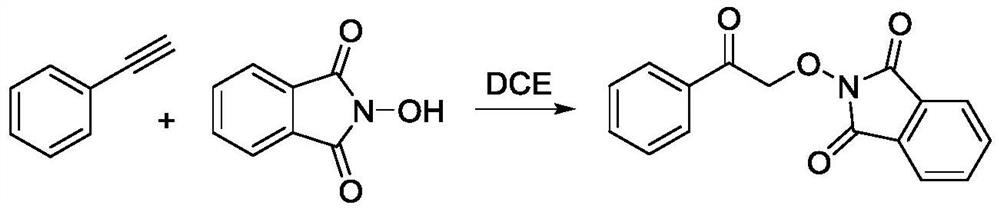
Method for preparing 2-(2-oxo-2-phenethoxy) isobenzyl-1, 3-diketone
A technology of phenylethoxy and oxo, which is applied in the field of oxidative alkylation reaction to achieve the effect of simple process, mild reaction conditions, and no catalyst and oxidant participation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 0.2mmol of N-hydroxyphthalimide, 0.2mmol of phenylacetylene and magneton into the reactor, feed oxygen, and then add 2ml of 1,2-dichloroethane under oxygen atmosphere, and react The device was placed in an oil bath at 25°C to heat the reaction, the magnetic stirrer speed was adjusted to 400-600 rpm, and the reaction was carried out for 8-16 hours. After the reaction was completed, the reaction solution was transferred to a separatory funnel, dichloromethane and water were added for extraction, and the crude product obtained by vacuum distillation of the organic phase was collected. Finally, the crude product was subjected to column separation (ethyl acetate:petroleum ether=1:10) to obtain 2-(2-oxo-2-phenylethoxy)isobenzyl-1,3-dione as a white solid product.
[0021] The structure of the product is determined by H NMR and C NMR: 1 H NMR (400MHz, CDCl 3 )δ8.21(d,J=8Hz,2H),7.95-7.93(m,2H),7.84-7.82(m,2H),7.71(m,1H),7.55(t,J 1 =8Hz,J 2 =8Hz,2H). 13 CNMR (100MHz, CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com