Dual specificity dimer, dual specificity dimer-drug conjugate and application of dual specificity dimer and dual specificity dimer-drug conjugate
A drug conjugate and bispecific technology, which is applied in the field of bispecific dimer-drug conjugates and bispecific dimers, can solve the problems such as the stability of receptor ligand binding needs to be improved, To achieve high-efficiency and low-toxicity treatment, reduce adverse reactions, and reduce dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
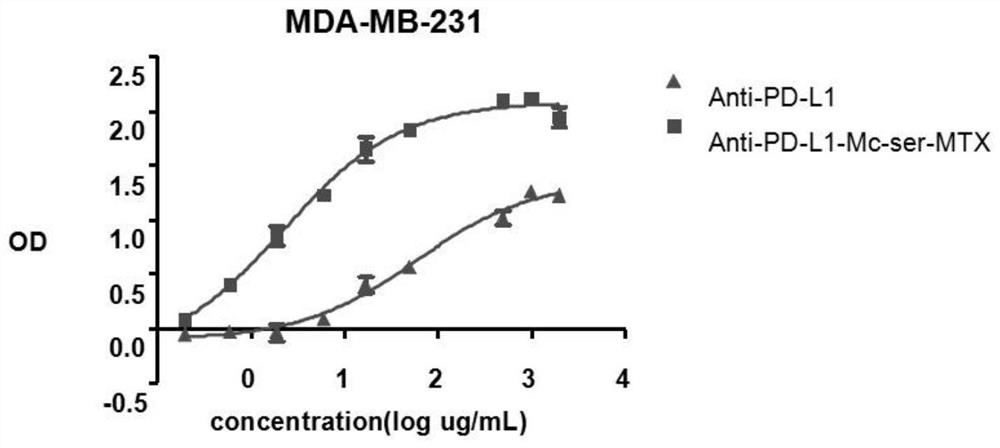
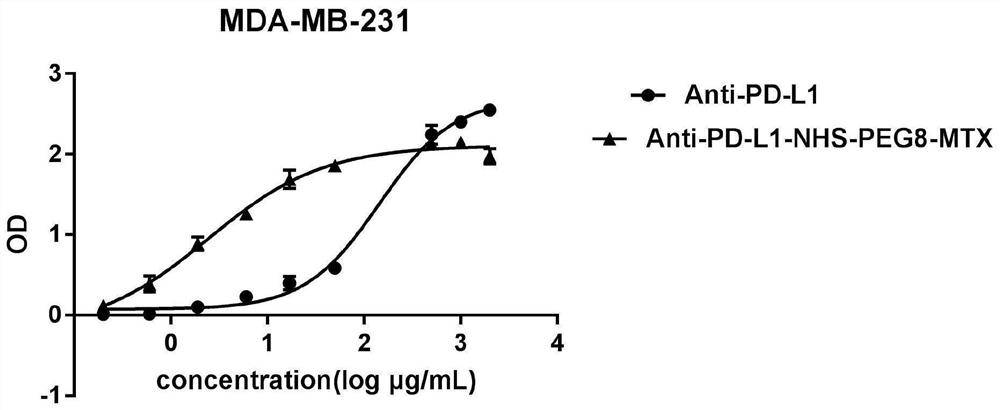
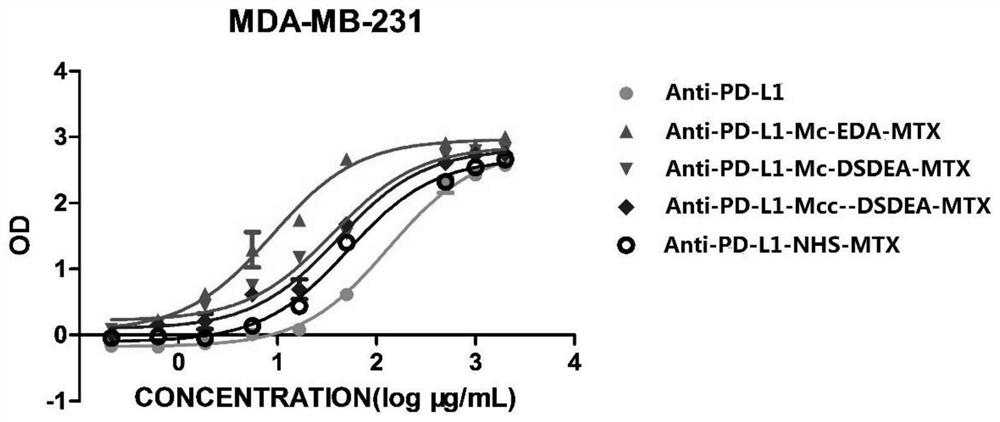
Image
Examples
Embodiment 1
[0052] Embodiment 1 compound I-1', I-2', I-3', I-4', I-5', I-6', I-7', I-8' (ie L 1 -L 2 -A 2 ) preparation
[0053] (1) Preparation of I-1'
[0054]
[0055]
[0056] N 2 Under protection, weigh MTX (227mg, 0.5mmol, purchased from Bailingwei Technology) into a 50mL one-mouth eggplant-shaped bottle, add TEA (101.2mg, 1mmol) and 3mL DMF, and stir at -5°C for 10min. Weigh TSTU (113.5mg, 0.55mmol), dissolve in 1mL DMF, add dropwise to the above reaction solution, and stir for 3h. Compound 1 (212mg, 0.5mmol) was weighed, dissolved in 2mL DMF, added dropwise to the above reaction solution, and stirred for 5h. After the reaction, the reaction solution was diluted with 3 mL of water, separated by preparative HPLC, and the prepared solution was freeze-dried to obtain 288.1 mg of yellow oily compound 2 with a yield of 67%. LC-MS (ESI + )861[M+H] + .
[0057]
[0058]
[0059] N 2 Under protection, compound 2 (288.1 mg, 0.34 mmol) was weighed into a 10 mL single-ne...
Embodiment 2
[0113] Example 2 General preparation method of bispecific dimer
[0114] Antibody (10mg / mL), DTPA (10mM) and 2.5eq. of TCEP (5mM) were added to the PCR tube, and the reaction was stirred at room temperature for 2h. Then add 25% DMSO and 5eq. of methotrexate-L in ice bath 1 (5mM) (such as I-1', I-2', I-3', I-4', I-5', I-6', I-7', I-8' and other compounds), stirring at room temperature Reaction 10h. After the reaction, centrifuge and ultrafilter three times with PBS buffer to purify and remove residual unreacted drugs and free small molecules such as DMSO.
[0115] We used the method described above to prepare the following bispecific dimers:
[0116]
[0117]
[0118]
Embodiment 3
[0119] Example 3 General Preparation Method of Bispecific Dimer-Drug Conjugate
[0120] Antibody (10mg / mL), DTPA (10mM) and 2.5eq. of TCEP (5mM) were added to the PCR tube, and the reaction was stirred at room temperature for 2h. Then add 25% DMSO and 5eq. of the corresponding linker-toxin in ice bath, and react for 2h. 2.5eq. of TCEP (5mM) was added, stirred at room temperature for 2h, and then linker-MTX (5mM) was added, stirred at room temperature for 10h. After the reaction, centrifuge and ultrafilter three times with PBS buffer to purify and remove residual unreacted drugs and free small molecules such as DMSO.
[0121] We used the above method to prepare the following bispecific dimer-drug conjugates:
[0122]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com