Benzyl-, (pyridin-3-yl)methyl- or (pyridin-4-yl)methyl-substituted oxadiazolopyridine derivatives as ghrelin O-acyl transferase (GOAT) inhibitors
A kind of technology of pyridine and substituent, applied in the field of novel diazolopyridine derivatives
Active Publication Date: 2020-09-11
BOEHRINGER INGELHEIM INT GMBH
View PDF12 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
To date, no receptors for UAG have been identified, but functional antagonism of AG has been demonstrated, at least in terms of its metabolic properties (W. Zhang et al., Endocrinology (2008) 149(9), 4710-4716)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
[0378] The following examples are used to further explain the present invention, but are not intended to limit the present invention.
[0379] The compounds described below have been characterized by their characteristic masses after ionization in a mass spectrometer and / or by their retention times on analytical HPLC.
[0380] HPLC method:
[0381] method 1: Column: Waters XBridge C18, 3x 30mm, 2.5μm
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Login to View More
Abstract
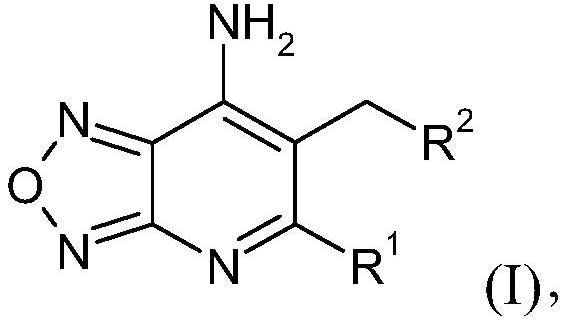
The present invention relates to compounds of general formula (I), wherein the groups R1 and R2 are defined as in claim 1, which have valuable pharmacological properties, in particular bind to ghrelinO-acyl transferase (GOAT) and modulate its activity. The compounds are suitable for treatment and prevention of diseases which can be influenced by this receptor, such as metabolic diseases, in particular obesity.
Description
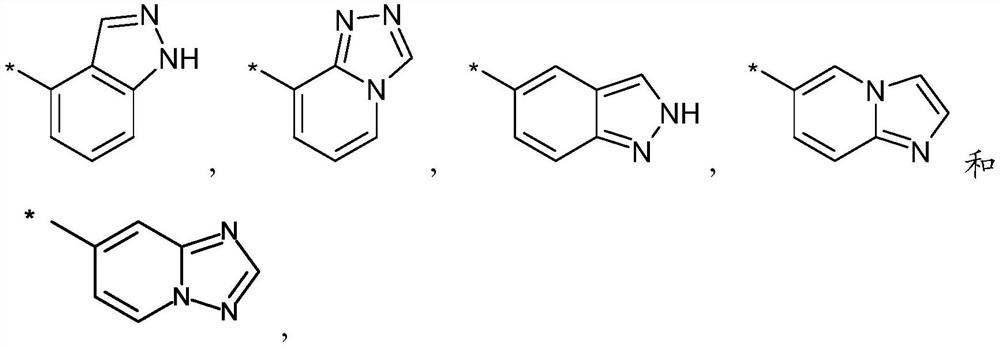
technical field [0001] The present invention relates to: new Oxadiazolopyridine derivatives, which are inhibitors of ghrelin O-acyltransferase (GOAT); preparation methods of these compounds; pharmaceutical compositions comprising these compounds; and their use in the prevention and / or treatment of ghrelin Medicinal use for diseases affected by the modulation of the function of O-acyltransferases (GOATs). In particular, the pharmaceutical composition of the present invention is suitable for the prevention and / or treatment of metabolic diseases, such as obesity, including but not limited to obesity, insulin resistance and diabetes in patients with Prader-Willi syndrome (PWS), in particular is type 2 diabetes. Background technique [0002] Ghrelin O-acyltransferase (GOAT) is a member of the membrane-embedded O-acyltransferase (MBOAT) protein family and the only enzyme in humans that can promote the acylation reaction of the peptide hormone ghrelin. By linking a medium-chain...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D498/04C07D519/00A61P3/00A61P3/10A61K31/437A61K31/444A61K31/5377A61K31/497A61K31/501A61K31/4545
CPCC07D498/04C07D519/00A61P3/00A61P3/10A61K31/437A61K31/444A61P3/04
Inventor T·特里塞尔曼C·哥德布C·霍克V·文顿雅克
Owner BOEHRINGER INGELHEIM INT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com