Application of dicoumarol in preparation of HBx protein stability inhibitor
A dicoumarin and inhibitor technology, which is applied in the direction of antiviral agents, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., can solve the problem of lack of treatment methods for hepatitis B, reduce stability and inhibit replication Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1 Cell Culture and Transfection
[0107] HepG2-NTCP cell line was cultured in DMEM medium containing 10% fetal bovine serum and 2.5 μg / mL puromycin; PHH cells were cultured in HM medium; Huh-7 cell line was cultured in DMEM medium containing 10% fetal bovine serum medium. All cells were in 5% CO 2 , Routine culture in a 37°C incubator. The plasmid was transfected according to the instructions of Lipofectamin 3000TM (Invitrogen).
Embodiment 2
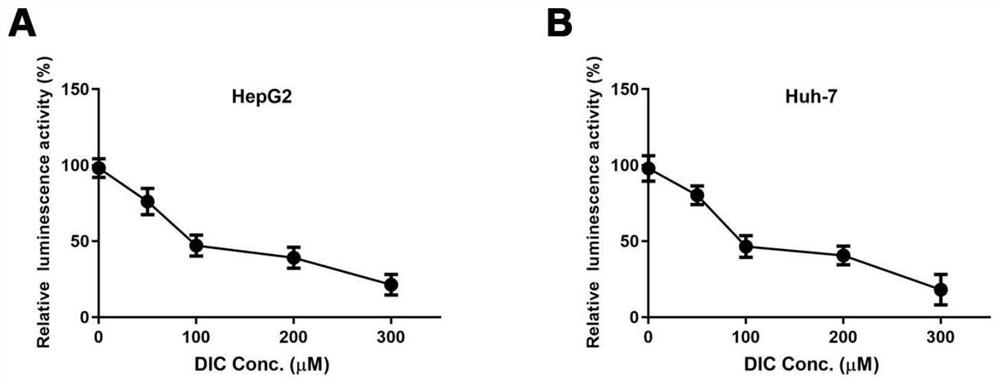
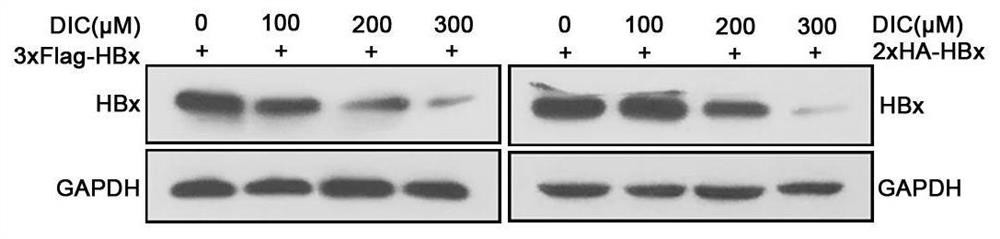
[0108] Example 2 HiBiT cleavage assay screened out that Dicoumarol can significantly inhibit the expression of HBx
[0109] In order to screen drugs targeting HBx inhibition, we constructed a drug screening model targeting HBx based on Promega's HiBiT cleavage detection system. First, the polypeptide HiBiT containing 11 amino acid residues is fused with HBx to obtain the HiBiT-HBx expression plasmid, which is transferred into HepG2 and Huh-7 cells, and then treated with candidate drugs;
[0110] The cells after drug treatment were taken out, washed twice with PBS, and operated in strict accordance with the instructions of the kit and protected from light. configuration HiBiT Lytic Reagent: Calculate the required total amount based on 100 μL per well, taking the amount of 1 well as an example, add 100 μL in sequence to a sterile EP tube HiBiT Lytic Buffer, 1 μL HiBiT LyticLgBiT Protein (1:100) and 2μL HiBiT Lytic Substrate (1:100), mix thoroughly; add 100μL to each we...
Embodiment 3
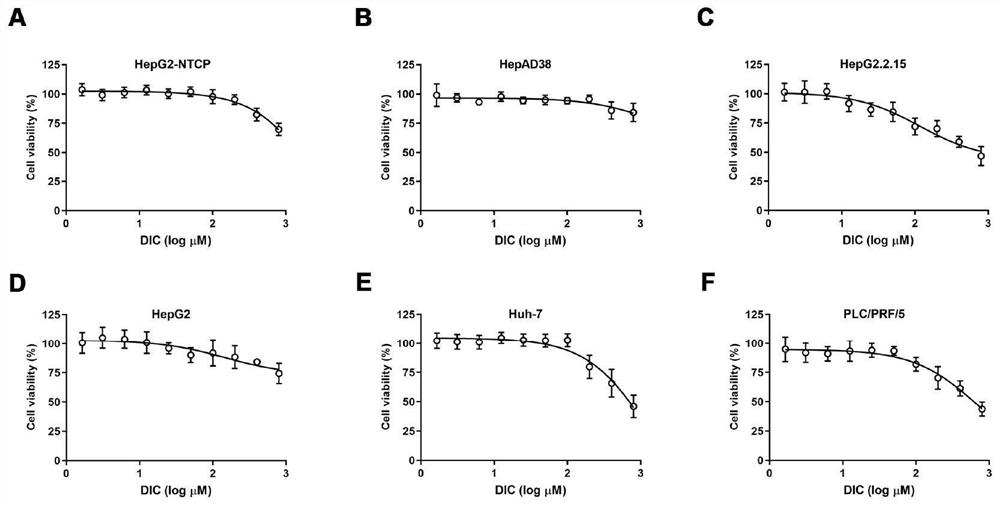
[0112] Example 3 MTT assay detects the cytotoxicity of Dicoumarol
[0113] In order to detect the cytotoxicity of the NQO1 inhibitor Dicoumarol, we first seeded different cells (including HepG2-NTCP, HepAD38, HepG2.2.15, HepG2, Huh-7 and PLC / PRF / 5) in 96-well plates, using different concentrations Dicoumarol (DIC) treated cells for 72 hours, specific operations:
[0114] Dissolve DIC with 0.13N NaOH to prepare a 40mM stock solution. will be 1.5×10 4 Huh-7 cells or HepG2-NTCP cells were inoculated in 96-well plates. After 24 hours, the DIC stock solution was diluted with growth medium to obtain 0 μM, 1.925 μM, 3.90 μM, 7.8125 μM, 15.625 μM, 31.25 μM μM, 62.5 μM, 125 μM, 250 μM, 500 μM, and 1000 μM DIC culture medium; and the no-drug treatment group was used as a control, and the pure medium was used as a blank. Each concentration was replicated in three wells, and 100 μl of the above solution was added to each well. After 72h, add 10ul of MTT reagent, incubate at 37°C for 4h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com