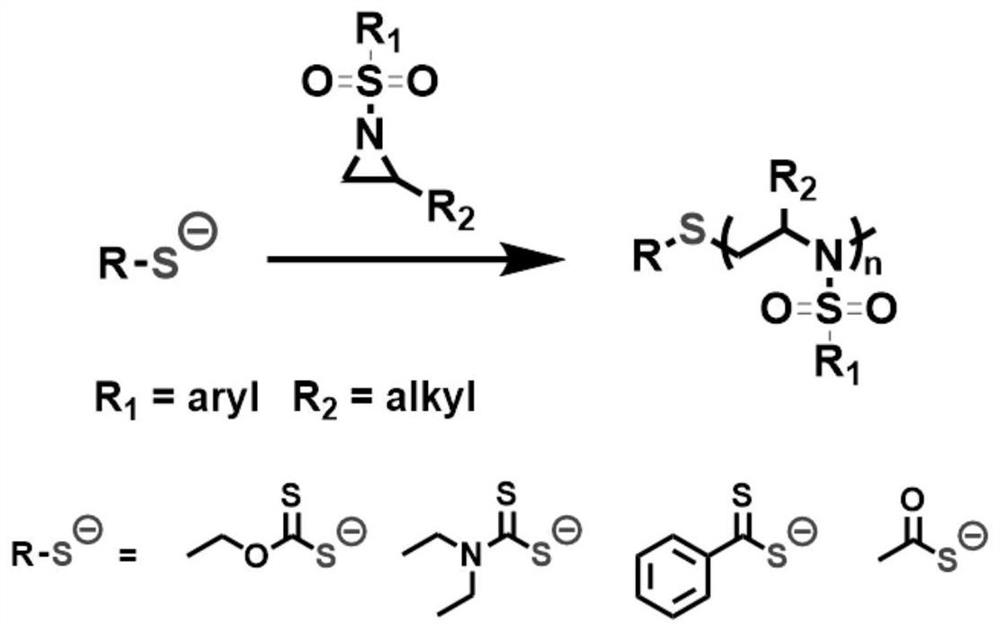
Air atmosphere anion ring-opening polymerization method of N-sulfonyl aziridine derivative
A heterocyclopropane, ring-opening polymerization technology, applied in the field of polymer chemistry, can solve the problems of unclear polymer structure and wide molecular weight distribution, and achieve the effect of wide variety, easy availability and variety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, Potassium ethyl xanthate initiates the air atmosphere polymerization of 2-methyl-N-tosyl aziridine
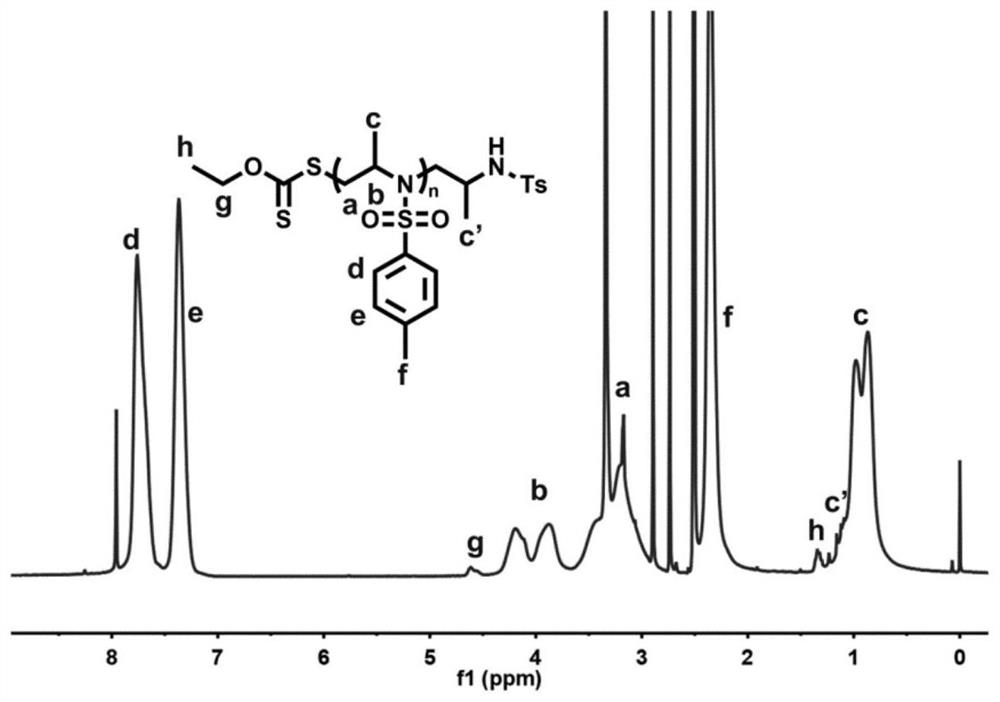
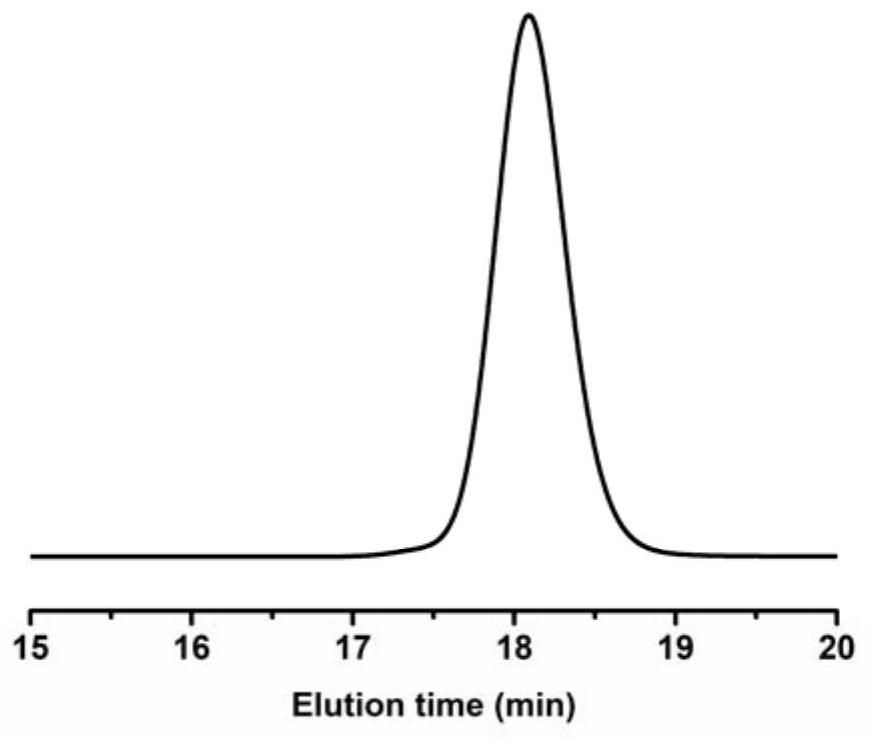
[0037] In an air atmosphere (relative humidity 70%), weigh 8.0 mg of potassium ethyl xanthate and 530 mg of 2-methyl-N-toluenesulfonyl aziridine, add them to the reaction tube, and then add dimethylformamide 2.5mL, stirred and mixed evenly, and reacted at 25°C for 4h (the synthetic route is as follows figure 1 shown). Precipitation in methanol yielded poly(2-methyl-N-tosylaziridine). GPC test molecular weight is 9500Da, PDI is 1.05.
[0038] figure 2 It is the NMR spectrum of the poly(2-methyl-N-tosylaziridine) prepared in Example 1.
[0039] image 3 It is the GPC elution curve of the poly(2-methyl-N-tosylaziridine) prepared in Example 1.
Embodiment 2
[0040] Embodiment 2, Potassium ethyl xanthate initiates the air atmosphere polymerization of 2-methyl-N-4-bromobenzenesulfonyl aziridine
[0041] In an air atmosphere (relative humidity 70%), weigh 8.0 mg of potassium ethyl xanthate, 690 mg of 2-methyl-N-4-bromobenzenesulfonyl aziridine, add them to the reaction tube, and then add dimethyl 2.5mL of methyl formamide, stirred and mixed evenly, and reacted at 25°C for 4h. Precipitate in methanol to obtain poly(2-methyl-N-4-bromobenzenesulfonylaziridine). GPC test molecular weight is 8500Da, PDI is 1.06.
[0042] Figure 4 It is the NMR spectrum of the poly(2-methyl-N-4-bromobenzenesulfonyl aziridine) prepared in Example 2.
[0043] Figure 5 GPC elution curve for the poly(2-methyl-N-4-bromobenzenesulfonylaziridine) prepared in Example 2.
Embodiment 3
[0044] Embodiment 3, Potassium ethyl xanthate initiates the air atmosphere polymerization of 2-methyl-N-4-nitrobenzenesulfonyl aziridine
[0045] In an air atmosphere (relative humidity 70%), weigh 4.0 mg of potassium ethyl xanthate and 305 mg of 2-methyl-N-4-nitrobenzenesulfonyl aziridine, add them to the reaction tube, and then add two Methylformamide 1.25mL, stirred and mixed evenly, reacted at 25°C for 4h. Precipitate in methanol to obtain poly(2-methyl-N-4-nitrobenzenesulfonylaziridine). GPC test molecular weight is 7800Da, PDI is 1.35.
[0046] Figure 6 It is the NMR spectrum of the poly(2-methyl-N-4-nitrobenzenesulfonyl aziridine) prepared in Example 2.
[0047] Figure 7 GPC elution curve for the poly(2-methyl-N-4-nitrobenzenesulfonylaziridine) prepared in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com