New application of n-butanol-2-o-(l-pyroglutamic acid-n-6-)-α-d-fructofuranoside
A technology of -N-6-, fructofuranoside, which is applied in the new application field of n-butanol-2-O--α-D-fructofuranoside, can solve the problem of improving the mechanism of AD that cannot delay the development of the disease Insufficient in-depth, unable to verify the effect of drug treatment and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. The specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0042] The new application of n-butanol-2-O-(L-pyroglutamic acid-N-6-)-α-D-fructofuranoside specifically includes the following contents.
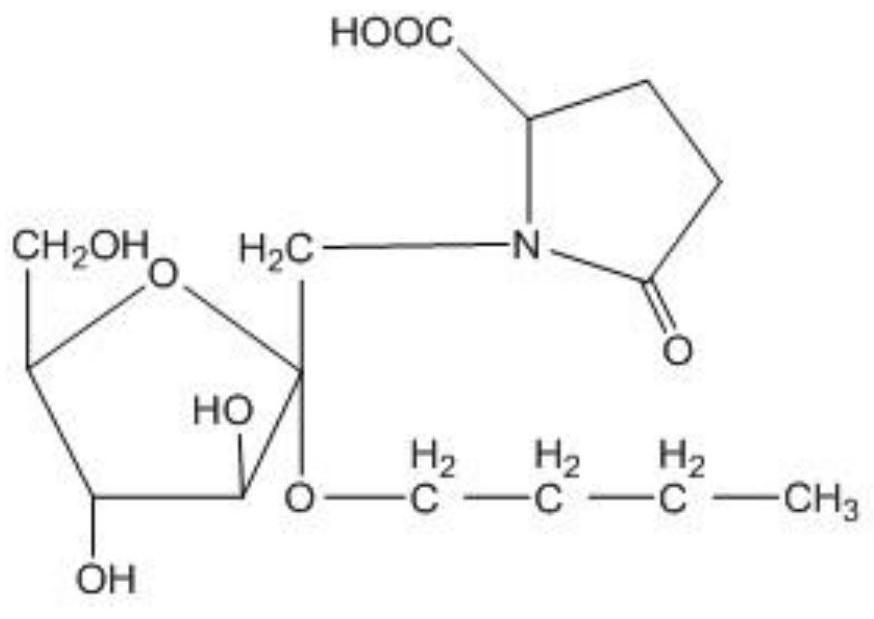
[0043] Such as figure 1 as shown, figure 1 It is the structural formula of n-butanol-2-O-(L-pyroglutamic acid-N-6-)-α-D-fructofuranoside, wherein, n-butanol-2-O-(L-pyroglutamic acid Amino acid-N-6-)-α-D-fructofuranoside C1 and C4 large groups on the fructose are located on both sides, that is, the glycosidic bond is α-type, that is, n-butanol-2-O-( L-pyroglutamic acid-N-6-)-α-D-fructofuranoside is referred to as L-pyroglutamic acid α.
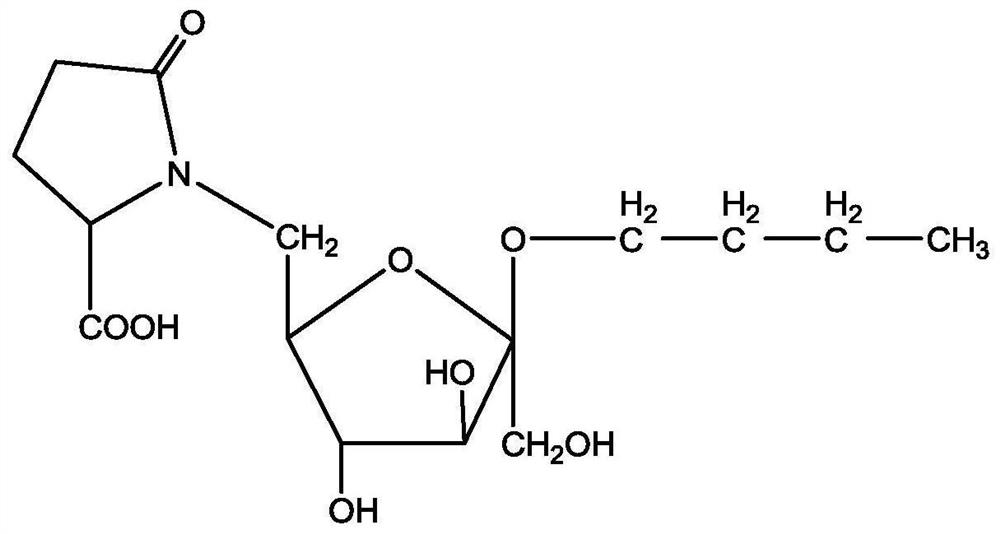
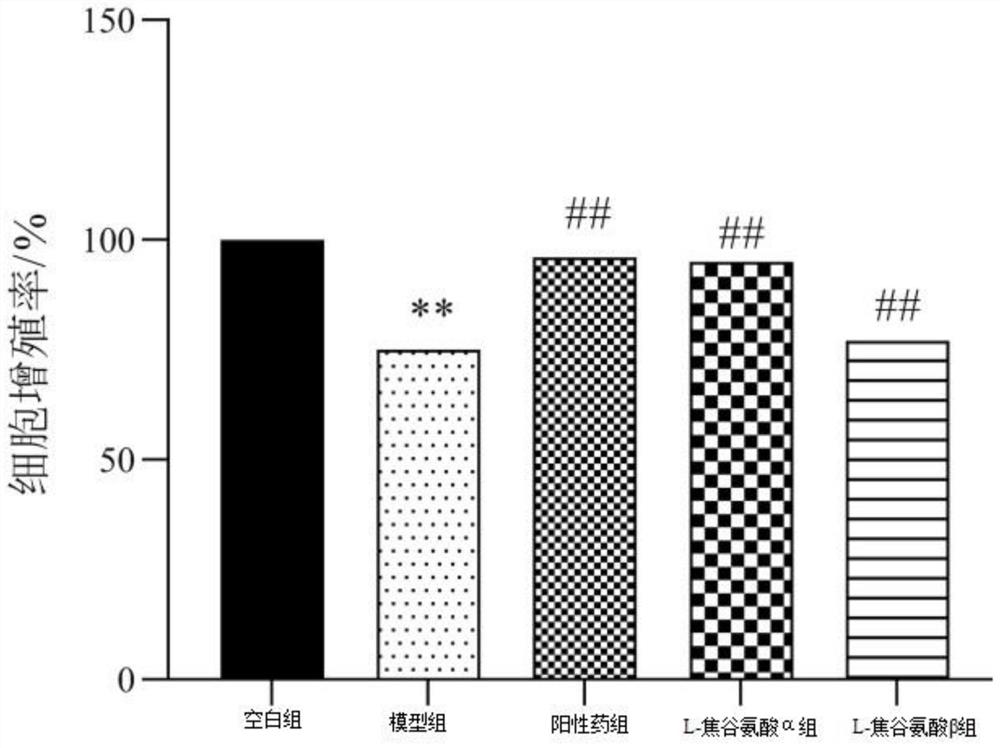
[0044] Such as figure 2 as shown, figure 2 It...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com