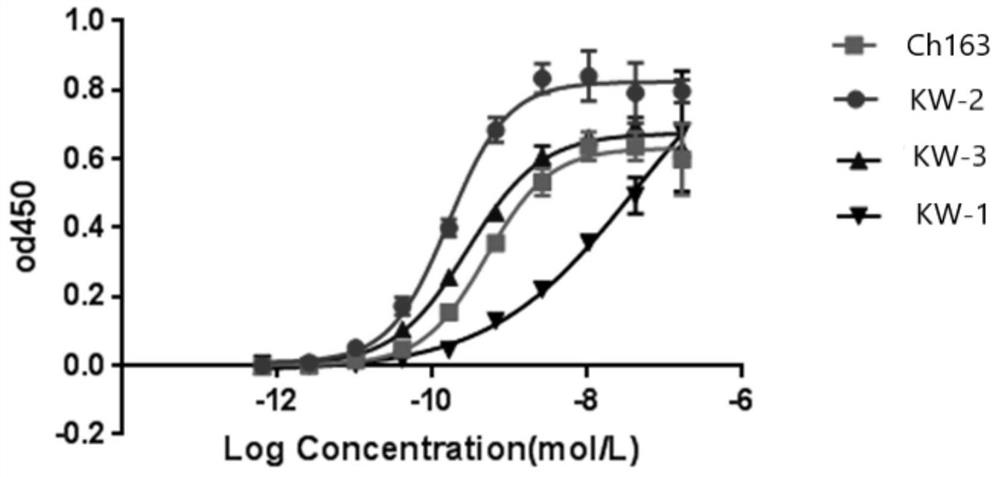
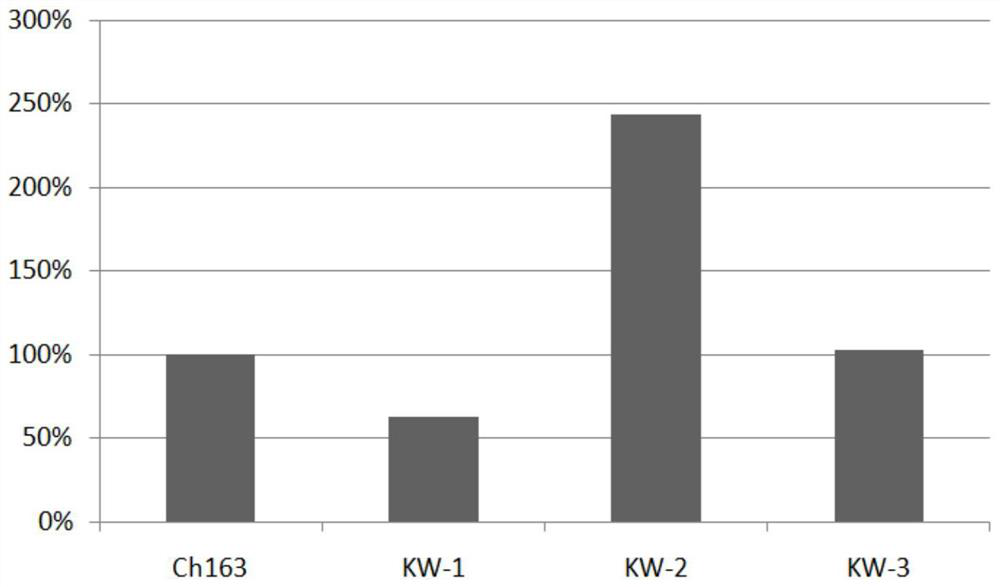
Anti-CLDN 18 fully-humanized antibody for treatment of late gastric cancer
A fully humanized, advanced gastric cancer technology, applied in the direction of antibodies, recombinant DNA technology, and the use of vectors to introduce foreign genetic materials, can solve the problems of reduced expression of intestinal type gastric cancer and Claudin18 expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Construction of HEK293 cells expressing Claudin18.1 and Claudin18.2 antigens
[0044] The pcDNA3.1 vector (purchased from Invitrogen) encoding human Claudin18.1 and Claudin18.2 antigens was transfected into HEK293 cells (purchased from ATCC), and 200 μg / mL geneticin was used as the selection pressure to obtain stable expression of Claudin18.1 and Claudin18.1. HEK293 cells with Claudin18.2 antigen. Using Ganymed’s Claudin18.2 antibody IMAB362 (self-made, transiently expressed in CHO-S cells, and further chromatographically purified, refer to Examples 2 and 3, denoted as ch163 in the present invention) as a positive antibody, the stable expression was screened by FACS method HEK293 cells with human Claudin18.1 and Claudin18.2 antigens.
Embodiment 2
[0045] Example 2 Construction of Candidate Antibody KW-2 Expression Vector
[0046] The HindIII restriction site ( AAGCTT ), KoZAK sequence ( GCCGCCACC ), ATG, signal peptide gene GAGAGAGACACACTCCTGCTATGGGTACTGCTGCTCTGGGTTCCAGGTTCCACTGGT and antibody heavy chain coding gene (including heavy chain variable region coding gene SEQ ID NO: 11 and constant region IgG1 coding gene SEQ ID NO: 17), termination code TAA and EcoRI coding gene GAATTC Sequential fusion in series, and the use of chemical synthesis to obtain gene fragments. Through the EcoRI and HindIII sites, the above fragment was inserted into the eukaryotic expression plasmid pCDNA 3.4(+) ((purchased from Invitrogen)) and verified by sequencing to obtain the expression plasmid pCDNA3.4(+)-BY6-4 of the antibody heavy chain .
[0047] The HindIII restriction site ( AAGCTT ), KoZAK sequence ( GCCGCCACC ), ATG, signal peptide gene GAGAGAGACACACTCCTGCTATGGGTACTGCTGCTCTGGGTTCCAGGTTCCACTGGT and antibody light chain cod...
Embodiment 3
[0050] Example 3 Expression and purification of anti-Claudin18.2 antibody
[0051] Using the DNA constructs described in Example 2, they were respectively transiently transferred to CHO-S cells (purchased from Invitrogen) to express the target antibody, according to the CHO-S cell operation manual (Freedom TM CHO-S TM KitUSER GUIDE), adjust the cell density to 1x10 the day before plasmid transfection 6 individual / ml. On the day of plasmid transfection, mix with the transfection reagent and add to EXPICHOEXPRESSION MEDIUM cell culture medium (purchased from Invitrogen Company), 37°C, 8% CO2 Continue to culture until the 8th day, collect the cell liquid, and remove the cells by centrifugation, 0.2 μm After filtration, Protein A affinity chromatography was used for purification, and the pH of the collected samples was adjusted to 5.5, and stored at 2-8°C. The purified antibody was detected by SDS PAGE and SEC, and the purity was above 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com