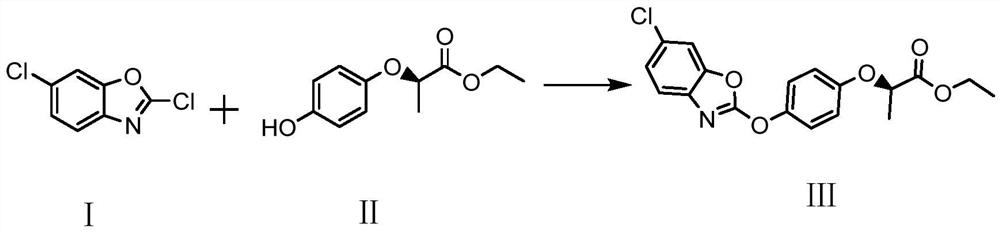
A kind of method of synthesizing herbicide fenoxafen
A technology for oxaflufen-ethyl and dichlorobenzoxazole, which is applied in the field of synthesizing herbicide oxaflufen-ethyl, can solve problems such as insufficient synthesis method and difficult waste water treatment, and achieves simple treatment and production cost saving. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 48.4g of acetone and 19.4g of 2,6-dichlorobenzoxazole to a 250ml three-necked flask, stir at a temperature of 20 to 25°C, and dissolve.
[0023] In a 250ml three-necked flask, add 29.5g of water, 21g of R-(+)-2-(4-hydroxyphenoxy)ethyl propionate, 31.8g of sodium carbonate, and 0.5g of benzyltriethylammonium chloride, stir, and heat up. to 60°C, and kept for 3h to obtain a mixed solution. The temperature was raised to 70°C, and the 2,6-dichlorobenzoxazole acetone solution was added dropwise to the mixture in the three-necked flask. The dropwise addition was completed for about 1 hour. During the reaction, the acetone in the system was evaporated, and the acetone aqueous solution was collected.
[0024] After the reaction is completed, keep the temperature of the system at 70-75°C, let stand for 30min to separate layers, separate the organic phase, add 30g of water to the organic phase, keep the temperature at 70-75°C, stir and wash for 30min, stand for 30min, separa...
Embodiment 2
[0026] Add 48.4g of acetone and 19.4g of 2,6-dichlorobenzoxazole to a 250ml three-necked flask, stir at a temperature of 20 to 25°C, and dissolve.
[0027] In a 250ml three-necked flask, add 29.5g of water, 21g of R-(+)-2-(4-hydroxyphenoxy)ethyl propionate, 26.5g of sodium carbonate, 0.5g of benzyltriethylammonium chloride, stir, and heat up to 60°C, and kept for 3h to obtain a mixed solution. The temperature was raised to 70°C, and the 2,6-dichlorobenzoxazole acetone solution was added dropwise to the mixture in the three-necked flask. The dropwise addition was completed for about 1 hour. During the reaction, the acetone in the system was evaporated, and the acetone aqueous solution was collected.
[0028] After the reaction is completed, keep the temperature of the system at 70-75°C, let stand for 30min to separate layers, separate the organic phase, add 30 g of water to the organic phase, keep the temperature at 70-75°C, stir and wash for 30min, stand for 30min, and separa...
Embodiment 3
[0030] 48.4g of acetone and 19.0g of 2,6-dichlorobenzoxazole were added to a 250ml three-necked flask, stirred at a temperature of 20-25°C, and dissolved.
[0031] In a 250ml three-necked flask, add 29.5g of water, 21g of R-(+)-2-(4-hydroxyphenoxy)ethyl propionate, 31.8g of sodium carbonate, and 0.5g of benzyltriethylammonium chloride, stir, and heat up. to 60°C, and kept for 3h to obtain a mixed solution. The temperature was raised to 70°C, and the 2,6-dichlorobenzoxazole acetone solution was added dropwise to the mixture in the three-necked flask. The dropwise addition was completed for about 1 hour. During the reaction, the acetone in the system was evaporated, and the acetone aqueous solution was collected.
[0032] After the reaction is completed, keep the temperature of the system at 70-75°C, let stand for 30min for layers, separate out the organic phase, add 30 g of water to the organic phase, keep the temperature at 70-75°C, stir and wash for 30min, stand for 30min, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com