Method and system for collecting clinical scientific research data
A technology for clinical scientific research and data collection, applied in the field of data processing, it can solve problems such as the inability to effectively collect clinical scientific research data, and the inability to meet actual requirements, so as to meet the needs of scientific research application scenarios, improve hospital scientific research capabilities, and reduce manual intervention.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
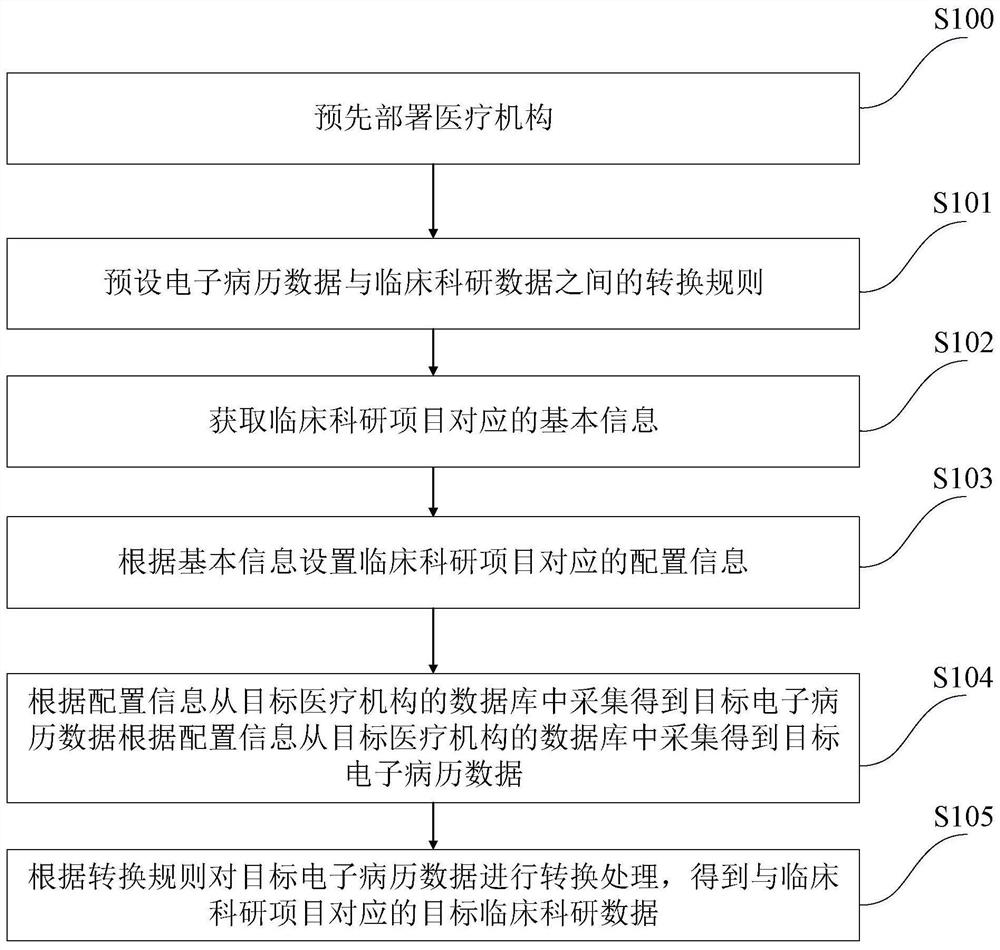
[0092] like figure 1 As shown, the collection method of the clinical scientific research data of the present embodiment comprises:
[0093] S100, pre-deploying medical institutions;
[0094] Specifically, according to the actual situation, a number of medical institutions within a certain area (province, city, county / district, etc.) are selected in advance as pilot medical institutions for the automatic collection of clinical scientific research data. Of course, the deployed medical institutions can also be adjusted according to actual needs.
[0095] The clinical data center of each pilot medical institution adopts the electronic medical record data model based on international information standards (HL7, ICD10, CDA, etc.) to ensure the standardized, structured and standardized collection of electronic medical record data of the pilot medical institutions.
[0096] Adopt the disease diagnosis-related group (DRG) grouping scheme suitable for the country or region, and comple...
Embodiment 2
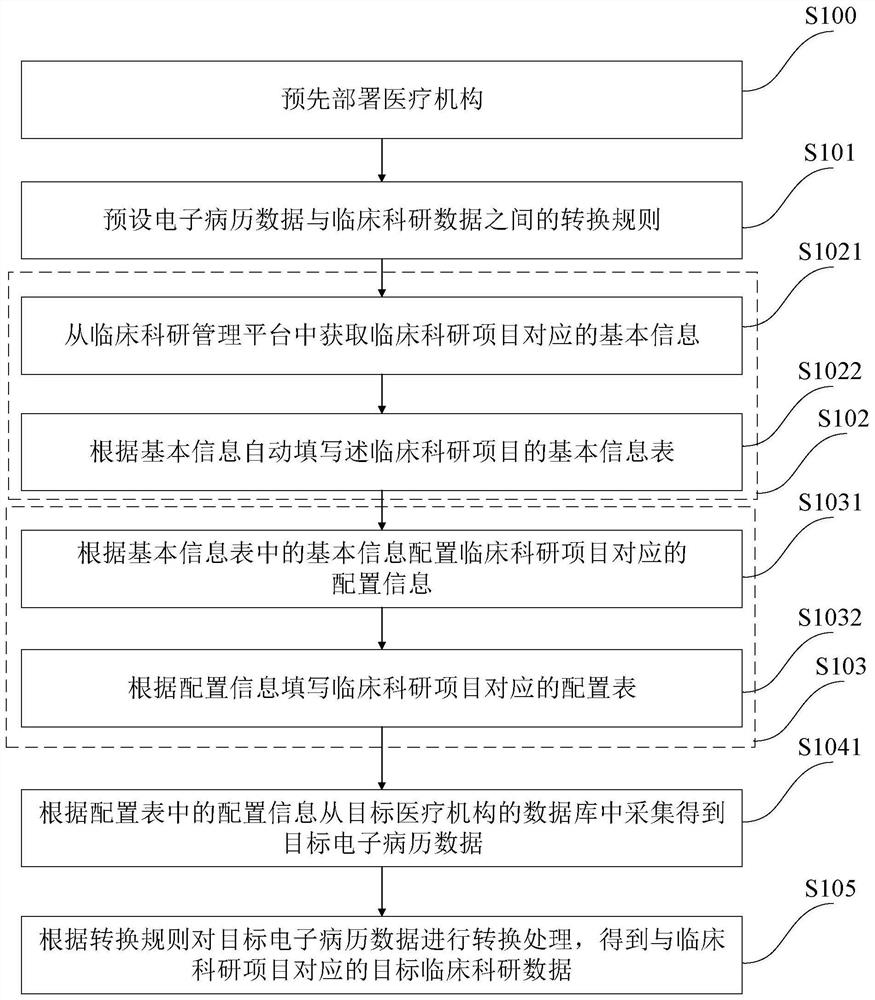
[0111] like figure 2 As shown, the collection method of clinical scientific research data in this embodiment is a further improvement to Embodiment 1, specifically:
[0112] Step S102 includes:
[0113] S1021. Obtain the basic information corresponding to the clinical scientific research project from the clinical scientific research management platform;
[0114]S1022. Automatically fill in the basic information form of the clinical scientific research project according to the basic information;
[0115] Step S103 includes:
[0116] S1031. Configure the configuration information corresponding to the clinical scientific research project according to the basic information in the basic information table;
[0117] S1032. Fill in the configuration form corresponding to the clinical scientific research project according to the configuration information;
[0118] After step S1022, before step S1031 also includes:
[0119] It is judged whether the information filled in the basic ...
Embodiment 3
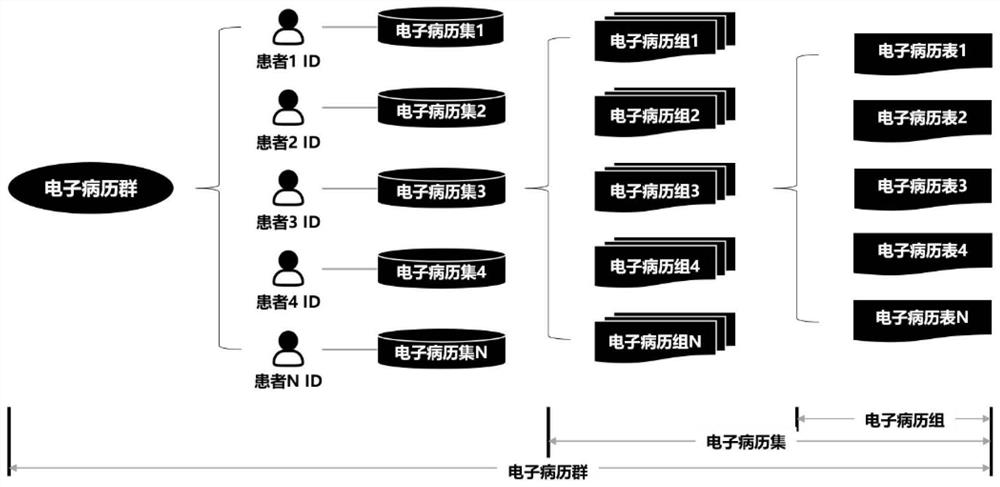
[0208] like Figure 5 As shown, the clinical scientific research data collection system of this embodiment includes a deployment module 1 , a rule preset module 2 , a basic information acquisition module 3 , an information setting module 4 , a collection module 5 and a conversion module 6 .
[0209] Deployment module 1 is used to pre-deploy medical institutions;
[0210] Specifically, according to the actual situation, a number of medical institutions within a certain area (province, city, county / district, etc.) are selected in advance as pilot medical institutions for the automatic collection of clinical scientific research data. Of course, the deployed medical institutions can also be adjusted according to actual needs.
[0211] The clinical data center of each pilot medical institution adopts the electronic medical record data model based on international information standards (HL7, ICD10, CDA, etc.) to ensure the standardized, structured and standardized collection of ele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com