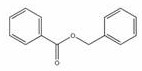
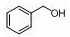
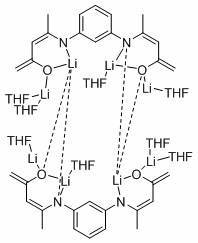
Application of deprotonated phenyl bridged beta-ketimine lithium compound in preparation of alcohol from ester
A technology of lithium ketimide and phenyl bridge is applied in the application field of preparing alcohol by hydroboration reaction. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under an inert gas atmosphere, 5.84 mg (0.005 mmol) of the catalyst was added to the reaction flask after dehydration and deoxygenation, and benzyl benzoate (94.9 μL, 0.5 mmol), pinacol borane (159.6 μL, 1.1mmol), THF (200 μL), at 60 o After reacting at C for 120 min, the reaction solution was exposed to air, and the solvent was removed under conventional reduced pressure to obtain the product boric acid ester, which was sampled and prepared for NMR, with mesitylene (69.6 μL, 0.5 mmol) as the internal standard, stirred evenly, and then added with dropwise Pipette a drop into the NMR tube, add CDCl 3 Dubbed into a solution, calculated 1 H spectrum yield was 96%. NMR data of the product: 1 HNMR (400 MHz, CDCl 3 ) δ 7.34 – 7.22 (m, 10H, ArH), 4.91 (s, 4H, OCH 2 ), 1.25 (s,24H, CH 3 ).
[0024] Add 1 g of silica gel and 2.5 mL of methanol to the system in which the solvent was removed after the hydroboration reaction, and react at 50°C for 2 hours. After the react...
Embodiment 2
[0030] Under an inert gas atmosphere, 5.84 mg of the catalyst was added to the reaction flask after dehydration and deoxygenation, methyl acetate (39.7 μL, 0.5 mmol), pinacol borane (159.6 μL, 1.1 mmol) were sequentially added with a pipette gun , THF (200μL), at 60 o After reacting at C for 120 min, the reaction solution was exposed to air, and the solvent was removed under conventional reduced pressure to obtain the product boric acid ester, which was sampled and prepared for NMR, with mesitylene (69.6 μL, 0.5 mmol) as the internal standard, stirred evenly, and then added with dropwise Pipette a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 95%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 3.87 (q, J = 7.1 Hz, 2H, OCH 2 ), 1.22 (s, 24H, OBpin), 1.19 (t, J = 6.0Hz, 3H, CH 3 ).
[0031] Add 1 g of silica gel and 2.5 mL of methanol to the system in which the solvent was removed after the hydroboration reaction,...
Embodiment 3
[0033]Under an inert gas atmosphere, add 5.84 mg (0.005 mmol) of the catalyst to the reaction flask after dehydration and deoxygenation, and add methyl p-bromobenzoate (107.52 mg, 0.5 mmol) and pinacol borane successively with a pipette gun. (159.6 μL, 1.1 mmol), THF (200 μL), at 60 o After reacting at C for 120 min, the reaction solution was exposed to air, and the solvent was removed under conventional reduced pressure to obtain the product boric acid ester, which was sampled and prepared for NMR, with mesitylene (69.6 μL, 0.5 mmol) as the internal standard, stirred evenly, and then added with dropwise Pipette a drop into the NMR tube, add CDCl 3 Dubbed into a solution, calculated 1 H spectrum yield was 98%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 7.40 (d, J = 8.3 Hz, 2H, ArCH), 7.17 (d, J = 8.2Hz, 2H, ArCH), 4.82 (s, 2H, OCH 2 ), 1.20 (s, 12H, OBpin), 1.19 (s, 12H, OBpin).
[0034] Add 1 g of silica gel and 2.5 mL of methanol to the system in which t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap