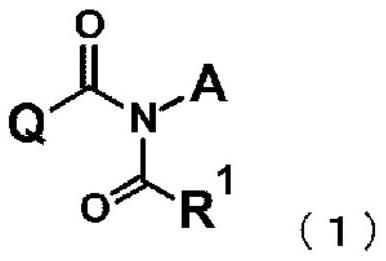
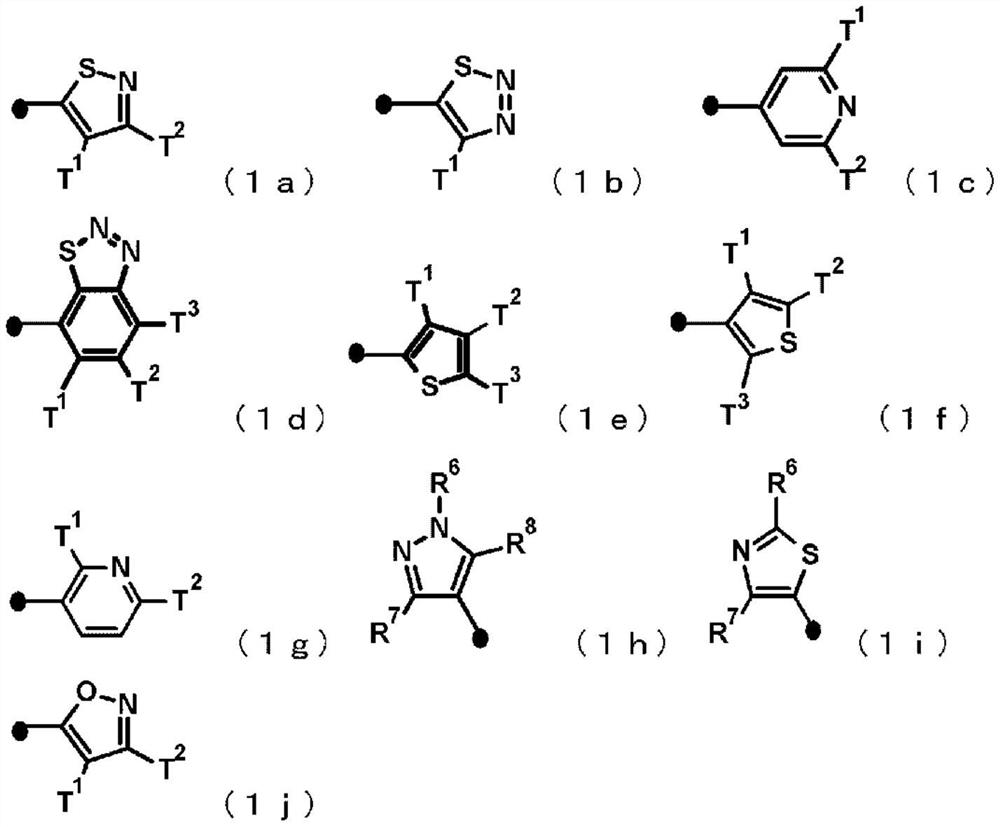
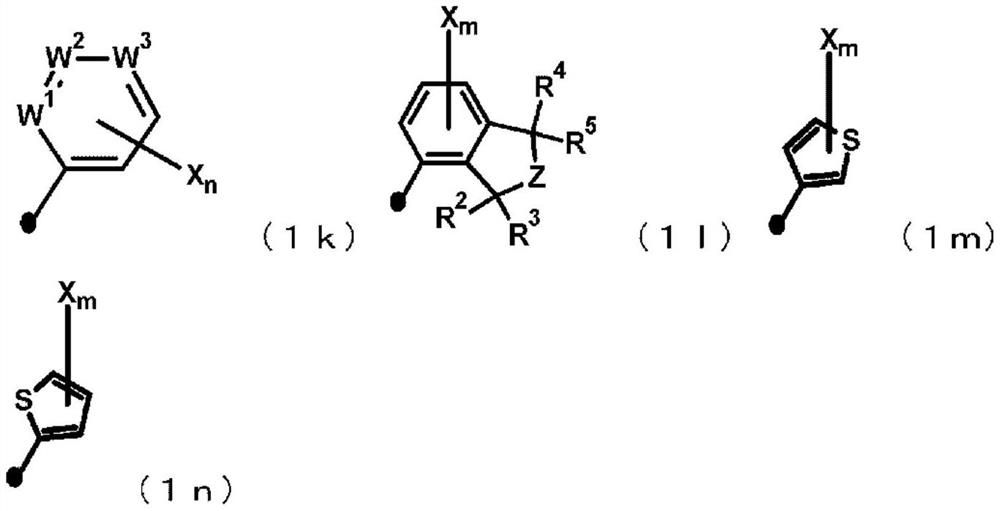
Imide derivative and bactericide containing same as active ingredient
A technology of derivatives and imides, applied in the field of imide derivatives, can solve the social requirements of reducing the amount of application or the amount of application, which has not been fully met, and the environmental pollution is unfavorable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0759] Hereinafter, the present invention will be described more specifically by referring to the synthesis examples, formulation examples and test examples of the compounds of the present invention, however, the present invention is not limited thereto in any way.
Synthetic example 1
[0761] N-{3-(Difluoromethyl)-1-methylpyrazole-4-carbonyl}-N-{2-(1,3-dimethylbutyl)phenyl}-4-methylthiadi Azole-5-carboxamide (number 1-146)
[0762] Thionyl chloride (619mg, 5.20mmol) and N,N-dimethylformamide (20mg, 0.27mmol) were added to the toluene solution of 4-methylthiadiazole-5-carboxylic acid (250mg, 1.73mmol) ( 10 mL) was heated to reflux for 2 hours, and the reaction mixture solution was concentrated to obtain 4-methylthiadiazole-5-carbonyl chloride.
[0763] The 3-(difluoromethyl)-N-{2-(1,3-dimethylbutyl)phenyl}-1-methyl-pyrazole-4-carboxamide (350mg) prepared in Reference Example 1 , 1.04 mmol) was added to a separate container containing sodium hydride (60%, 45.9 mg, 1.15 mmol) and tetrahydrofuran (2 mL), and the mixture was stirred at 25°C for 20 minutes. The tetrahydrofuran solution (2 mL) of 4-methylthiadiazole-5-carbonyl chloride prepared above was added thereto at 0°C, and the resulting mixture was stirred at 25°C for 1 hour. After confirming the completion of...
Synthetic example 2
[0765] 4-Difluoromethyl-N-{4-(difluoromethyl)-2-methylthiazole-5-carbonyl}-N-{2-(1,3-dimethylbutyl)phenyl}- 2-Methylthiazole-5-carboxamide (No. 7-238)
[0766] Thionyl chloride (620mg, 5.12mmol) and N,N-dimethylformamide (20mg, 0.27mmol) were added to 4-(difluoromethyl)-2-methyl-thiazole-5-carboxylic acid (500mg , 2.59 mmol) in toluene solution (10 mL), heated under reflux for 1 hour, and concentrated the reaction mixture solution to obtain 4-(difluoromethyl)-2-methyl-thiazole-5-carbonyl chloride.
[0767] The 4-(difluoromethyl)-N-{2-(1,3-dimethylbutyl)phenyl}-2-methylthiazole-5-carboxamide prepared in Reference Example 2 (700 mg, 1.99 mmol) was added to a separate container containing sodium hydride (60%, 87.4 mg, 2.19 mmol) and tetrahydrofuran (2 mL), and the mixture was stirred at 25°C for 20 minutes. The tetrahydrofuran solution (2 mL) of 4-(difluoromethyl)-2-methylthiazole-5-carbonyl chloride prepared above was added thereto at 0°C, and the resulting mixture was stirred at 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com