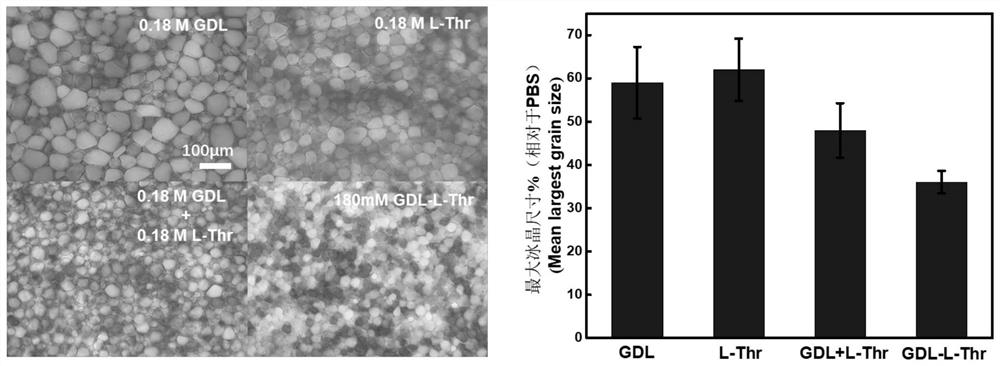
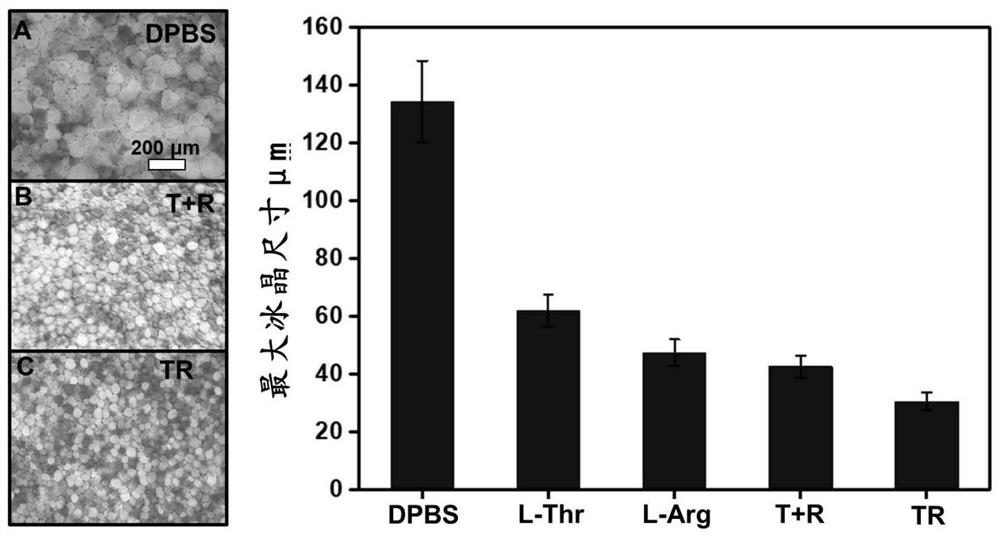
Peptide compound and cryopreservation solution containing same
A technology of cryopreservation liquid and peptide compounds, which is applied in the preservation of human or animal body, peptides, animal husbandry, etc., can solve the problems of lack of ice crystal growth, inability to effectively control ice crystal growth, damage to cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The preparation method of the present invention will be further described in detail in conjunction with specific examples below. It should be understood that the following examples are only for illustrating and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies realized based on the above contents of the present invention are covered within the scope of protection intended by the present invention.
[0080] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples can be obtained from commercial sources unless otherwise specified.
Embodiment 1
[0081] Synthesis of embodiment 1 formula (1) compound
[0082] (1) Put 2-chlorotrityl chloride resin (2-Chlorotrityl Chloride Resin) into the reaction tube, add DCM (20mL g -1 ), shaking for 30 minutes. The sand core was filtered to remove the solvent, and a three-fold molar excess of Fmoc-L-Pro-OH was added, followed by an eight-fold molar excess of DIEA, and finally DMF was added to dissolve, and shaken for 30 minutes. Methanol capping for 30 minutes.
[0083] (2) Remove the solvent DMF, add 20% piperidine / DMF solution (10mL g -1 ), after 5 minutes the solvent was removed, and a 20% piperidine / DMF solution (10 mL g -1 ), the piperidine solution was removed after 15 minutes. Take a small amount of resin, wash it three times with ethanol, add ninhydrin reagent, heat at 105-110°C for 5 minutes, if it turns dark blue, it is a positive reaction.
[0084] (3) The product obtained by the above reaction was washed with DMF (15mL g -1 , twice), methanol (15mL g -1 , twice) and...
Embodiment 2
[0090] Synthesis of embodiment 2 formula (2) compound
[0091] (1) Put 2-chlorotrityl chloride resin into the reaction tube, add DCM (20mL g -1 ), shaking for 30 minutes. The sand core was filtered to remove the solvent, and a three-fold molar excess of Fmoc-L-Thr(tBu)-OH was added, followed by an eight-fold molar excess of DIEA, and finally DMF was added to dissolve, and shaken for 30 minutes. Methanol capping for 30 minutes.
[0092] (2) Remove the solvent DMF, add 20% piperidine / DMF solution (10mL g -1 ), after 5 minutes the solvent was removed, and a 20% piperidine / DMF solution (10 mL g -1 ), the piperidine solution was removed after 15 minutes. Take a small amount of resin, wash it three times with ethanol, add ninhydrin reagent, heat at 105-110°C for 5 minutes, if it turns dark blue, it is a positive reaction.
[0093] (3) The product obtained by the above reaction was washed with DMF (15mL g -1 , twice), methanol (15mL g -1 , twice) and DMF (15mL g -1 , twice) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com