Eurotium cristatum product and applications thereof
A technology of coronoid spores and spores of spores, applied to medical preparations containing active ingredients, plant/algae/fungus/moss components, plant raw materials, etc., which can solve adverse side effects, single effect, and ineffective curative effect Good and other questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of the hyphae and spore of embodiment 1 coronoid saccharomyces
[0032] The taxonomy of the coronoid used in this embodiment is named Eurotium cristatum, which was preserved in the General Microorganism Center of China Microbiological Culture Collection Management Committee on April 2, 2018, and the preservation number is CGMCC NO.15398. It is also from the Isolated from Fu tea.
[0033] (1) Preparation of Mycelia of Mycelia coronoidis
[0034] According to the inoculum amount of 8%-10%, inoculate P. coronoidis in the fermenter containing 10L zinc-rich liquid submerged fermentation medium, wherein, the medium is: glucose 25g / L, peptone 2g / L by mass volume ratio , corn steep liquor dry powder 2.5g / L, yeast powder 10g / L, KH 2 PO 4 2g / L, MgSO 4 7H2O 4.1g / L, (NH4) 2 SO4 5g / L, sodium zincite 33.3mg / L, culture temperature 28-30℃, pH 5.0-5.5, air flow 5-20L / min, culture for 96h, collect the hyphae in the fermentation product, put it in Dry at 60-65°C for...
Embodiment 2
[0039] Preparation of Example 2 Coronoid Saccharomyces coronoidis product-capsule
[0040] The mycelia and spores of the Mycelia coronoidis prepared in Example 1 were used to prepare the capsule form of the Mycocystis coronoidis product according to different ratios. Specifically, the following 5 different capsules were prepared according to different proportions.
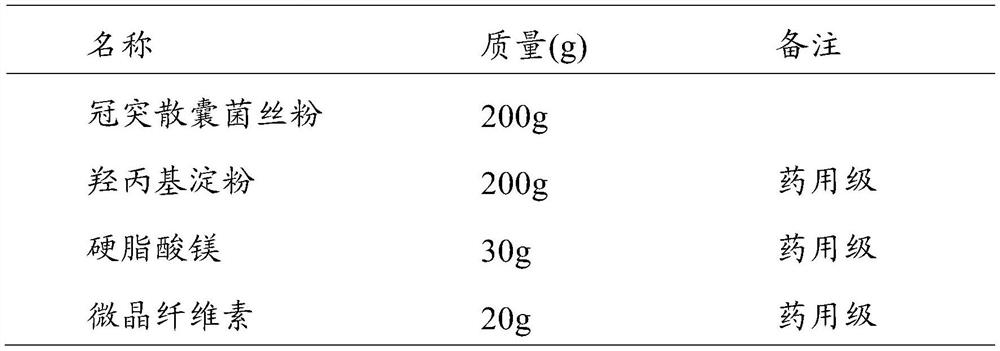
[0041] (1) Make capsules according to the ratio in Table 1 (1000 capsules, the content of S. coronoidis 200mg / capsule).
[0042] Table 1 The composition ratio of Coronary cyst capsules
[0043]
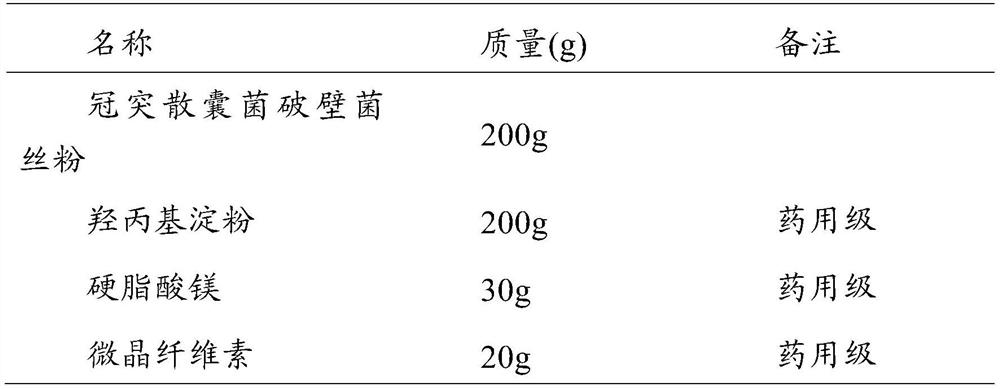
[0044](2) Capsules (1000 capsules, 200 mg / capsule of S. coronoidis content) were made according to the proportioning in Table 2.
[0045] Table 2 The composition ratio of Coronary cyst powder capsules
[0046]
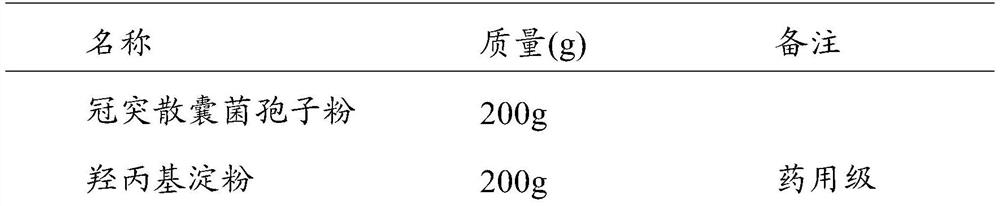
[0047] (3) Make capsules according to the proportioning in Table 3 (1000 capsules, the content of S. coronoidis 200 mg / capsule).
[0048] Table 3 The composition ratio of Coronary cyst capsules
[0049] ...
Embodiment 3
[0058] Preparation of Example 3 Coronoid Saccharomyces coronaris products-tablet
[0059] According to the proportioning of Table 6, tablet (1000 tablets, 200mg / tablet of S. coronoidis content) is made.
[0060] Table 6 The component distribution ratio of Cystis coronaris
[0061]
[0062] Specifically, during preparation, pass the above-mentioned main ingredients and auxiliary materials through 80-mesh sieves, mix well, use 75% ethanol as a binder, granulate with 16-mesh sieves, dry at 55-60 ° C, and granulate with 14-mesh sieves. Granules, compressed tablets, 0.3g per tablet.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap