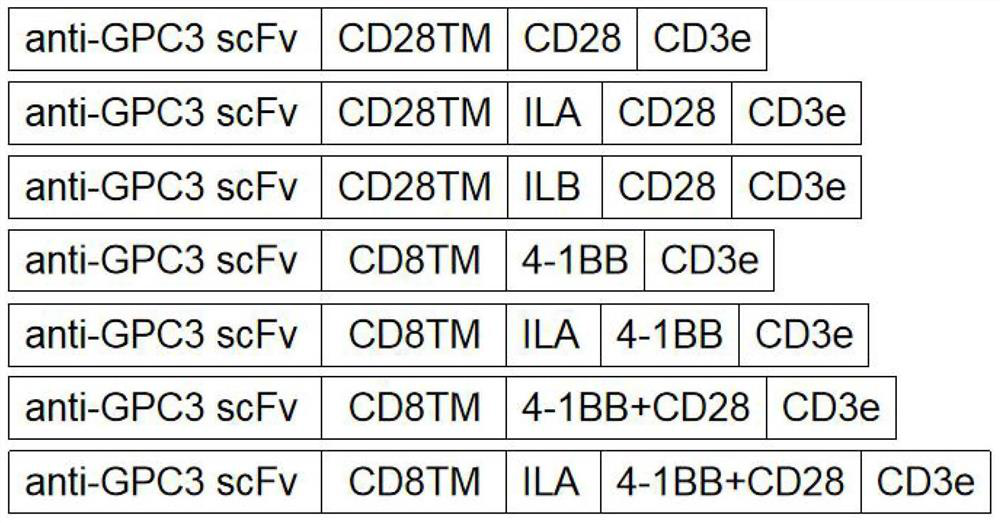
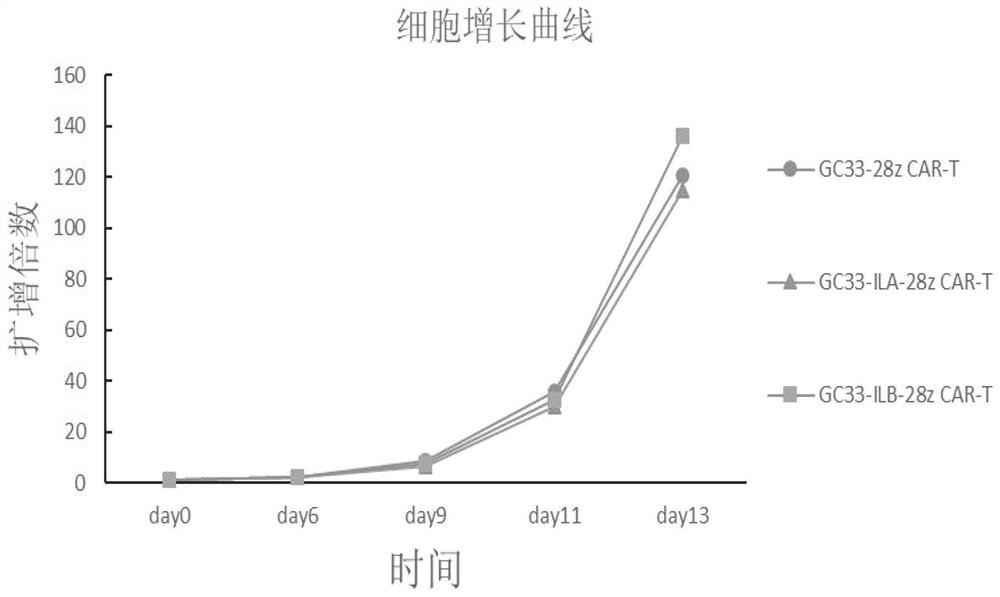
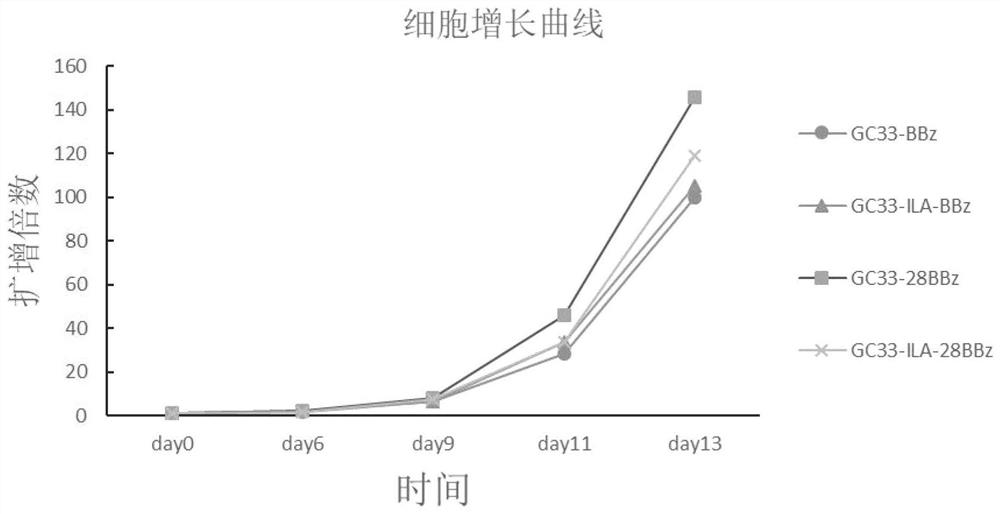
Immune cell and preparation and application thereof
A technology of immune cells and preparations, applied in the field of preparations and applications of immune cells, which can solve the problems of short survival time, low lethality, and less effective effect than hematological tumors, and achieve the effect of lasting time and reducing exhaustion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The preparation method and functional verification of immune cells in this embodiment include the following steps:
[0094] Step 1: Isolation of peripheral blood PBMCs and expansion of CAR-T cells
[0095] Mononuclear cells were isolated from donor peripheral blood, density gradient centrifugation was performed using ficol, and T cells were enriched with a T cell sorting kit (CD3 MicroBeads, human-lyophilized, 130-097-043), using a conjugated anti-CD3 / anti-CD28 magnetic beads activated culture and expansion of T cells; the medium used TexMACS GMP Medium (MiltenyiBiotec, 170-076-309), containing 10% FBS, 2mM L-glutamine, 100IU / ml rhIL2, all cells were placed Cultured in a constant temperature incubator at 37°C with 5% CO2.
[0096] Step 2: Cell Line Culture
[0097] Cell line expressing GPC3: Huh-7 (human liver cancer cell), purchased from ATCC.
[0098] Cell line not expressing GPC3: A549 (human non-small cell lung cancer cells), purchased from ATCC.
[0099] Cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com