Chimeric antigen receptor and application thereof
A chimeric antigen receptor, single-chain antibody technology, applied in the direction of antibody medical components, carriers, anti-tumor drugs, etc., can solve the problems of high recurrence rate, neurotoxicity, poor persistence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Embodiment 1 chimeric antigen receptor virus packaging
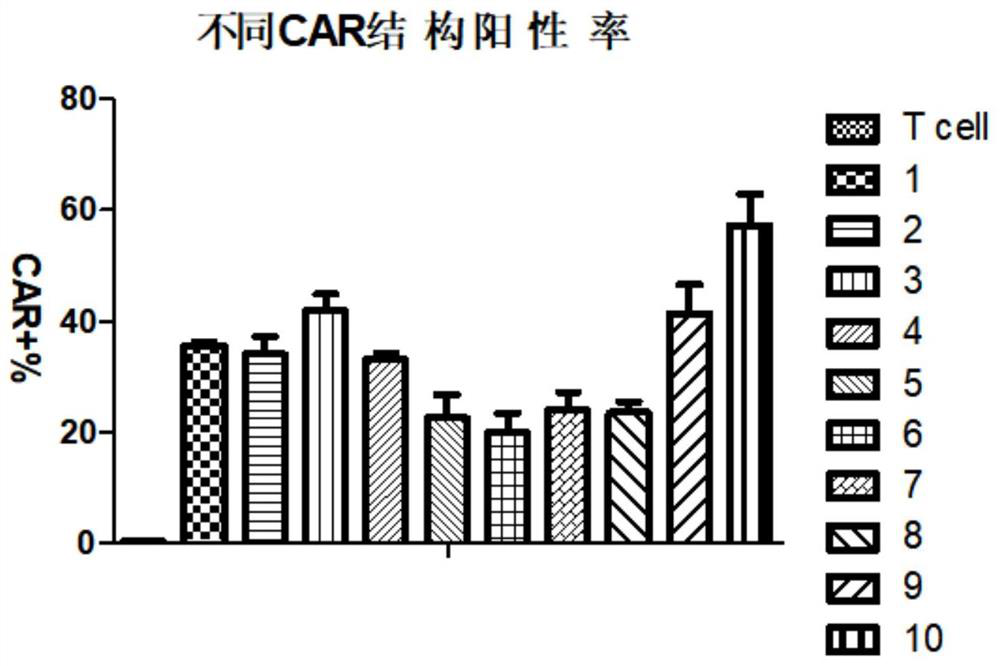
[0130] 1. Synthesis of CARs with different structures. The structures of the constructed CARs with signal peptides are shown in Table 1 below.
[0131] Table 1:
[0132]
[0133] Among them, the amino acid sequence of each part of the CAR structure with the signal peptide and the nucleotide sequence of the corresponding encoding nucleic acid are as follows:
[0134] The nucleotide sequence of the signal peptide (GL) signal peptide:
[0135] Atgggcgtcaaggtcctgttcgccctgatctgcatcgccgtcgccgaggcc
[0136] Amino acid sequence of signal peptide signal peptide (GL):
[0137] MGVKVLFALICIAVAEA
[0138] Nucleotide sequence of CD8 hinge region (hinge):
[0139] ACCACGACGCCAGCGCCGCGACCACCAACACCGGCGCCCACCATCGCGTCGCAGCCCCTGTCCCTGCGCCCAGAGGCGTGCCGGCCAGCGGCGGGGGGCGCAGTGCACACGAGGGGGCTGGACTTCGCCTGTGAT
[0140] Amino acid sequence of CD8 hinge region (hinge):
[0141] TTTPAPRPPTPAPTIASQPLSLRPEACRPAAGGAVHTRGLDFACD
[0142] T...
Embodiment 2
[0268] 1. T cell activation and lentivirus infection:
[0269] Take CD3 + Positive cells (viability 98.5%, living cells 5X10 6 ) 2mL, added to a 15mL centrifuge tube containing 5mL AIM-V, centrifuged at 500g for 5min to collect the cells and discard the supernatant, resuspended the cells with 10mL AIM-V medium (containing 20IU / mL IL-2) and transferred to 10cm Culture in a plate, take 180μL CD3 / 28 magnetic beads, add 4mL buffer to wash, put them in the magnetic stand for 2min, remove the supernatant, take 180μL AIM-V medium (containing 20IU / mL of IL-2) and resuspend, Add it to the cell solution, mix well, put it into an incubator for cultivation, and carry out virus transfection after 24 hours of cultivation.
[0270] 2. Take out the cells and place them in a 15mL centrifuge tube, place them in the magnetic stand for 2 minutes, and suck out the cell solution into another clean 15mL centrifuge tube. Take 10 μL of cell liquid, add 10 μL of AO / PI staining solution (staining sol...
Embodiment 3
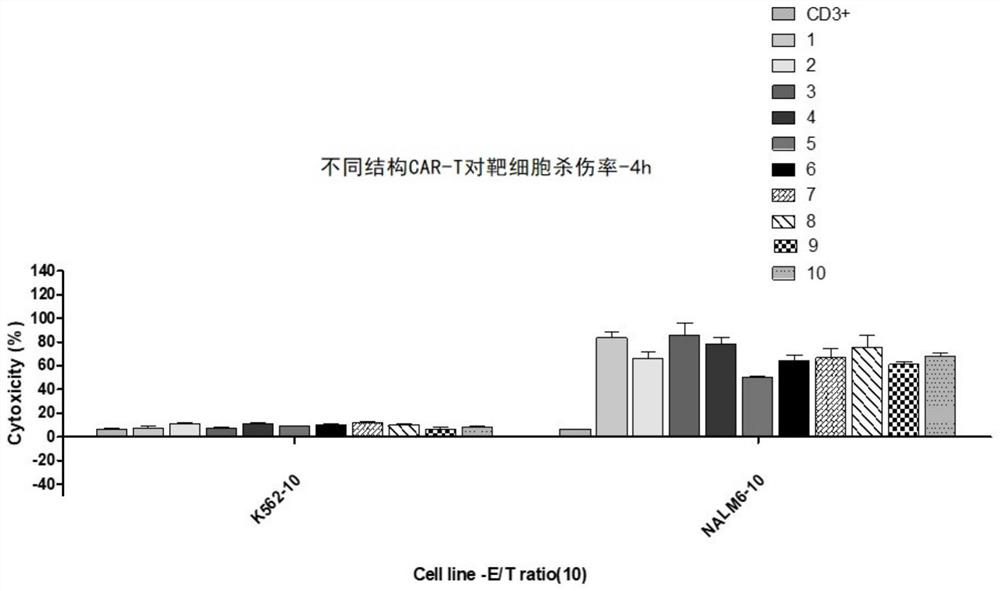
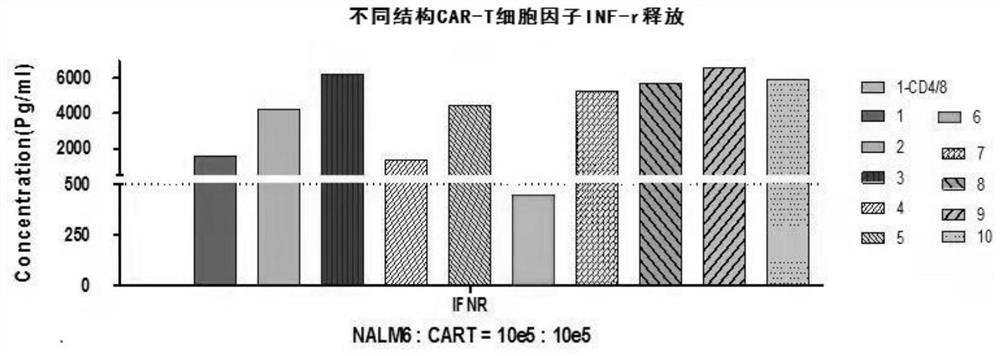
[0282] Detection of the killing effect of CD 19 CAR-T with different structures
[0283] 1. Cell culture
[0284] The effective target cells (NALM-6 / K562) were cultured in 1640 / IMDM+10% FBS medium, and cultured in a 37°C, 5% carbon dioxide incubator. Suspension cells: Use an inverted microscope to observe the cells once a day, and change the culture medium every 2-4 days. Cell growth density in culture flasks up to 3x10 6 before subculture of cells.
[0285] 2. Treatment of effector cells (CD 19 CAR-T with different structures)
[0286] Collect CAR-T cells with different structures into 15mL centrifuge tubes and centrifuge at 500g for 5min. Discard the supernatant, add 4 mL of 0.01M PBS to wash once, centrifuge to remove the supernatant, and then add an appropriate amount of AIM-V medium to resuspend to keep the density at 10 7 cells / mL or so. Determine cell density and viability.
[0287] 3. Pretreatment of target cells
[0288] 1) Labeling of target cells: Collect ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com