A kind of preparation method of oclatinib maleate
A technology of formula and cyclohexane carboxylic acid, which is applied in the field of chemistry or medicinal chemistry, can solve the problems of low utilization rate of raw materials and excessive solid waste, and achieve the effect of reducing post-processing times, increasing conversion rate, and avoiding processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] trans-4-(Methyl{7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidin-4-yl}amino)cyclohexyl]methylsulfinate Preparation of:
[0052] Add 350 g of toluene and 48.7 g (0.2 mol) of trans-4-[(tert-butoxycarbonyl)amino]cyclohexanecarboxylic acid to a 1L four-necked flask, and add 70% dihydrobis(2-methoxyethyl) dropwise. 202.2 g (0.7 mol) of oxy)sodium aluminate toluene solution was heated up to 110° C. and kept for 6 hours for reaction. Add 320 g (0.4 mol) of 5% sodium hydroxide solution to quench the reaction, separate the water layer, and concentrate the toluene layer to dryness. Add 200g of acetonitrile, 58.5g (0.19mol) of 4-chloro-7-toluenesulfonyl-7H-pyrrolo[2,3-d]pyrimidine, 54.3g (0.42mol) of N,N-diisopropylethylamine and Potassium iodide 3.7g (0.022mol), start stirring and raise the temperature to 65°C, and keep the reaction for 28 hours. The temperature was lowered to 5-10°C, and 34.4 g (0.3 mol) of methanesulfonyl chloride was added dropwise. After the additio...
Embodiment 2
[0055] Preparation of trans-4-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclohexyl)methanesulfonic acid:
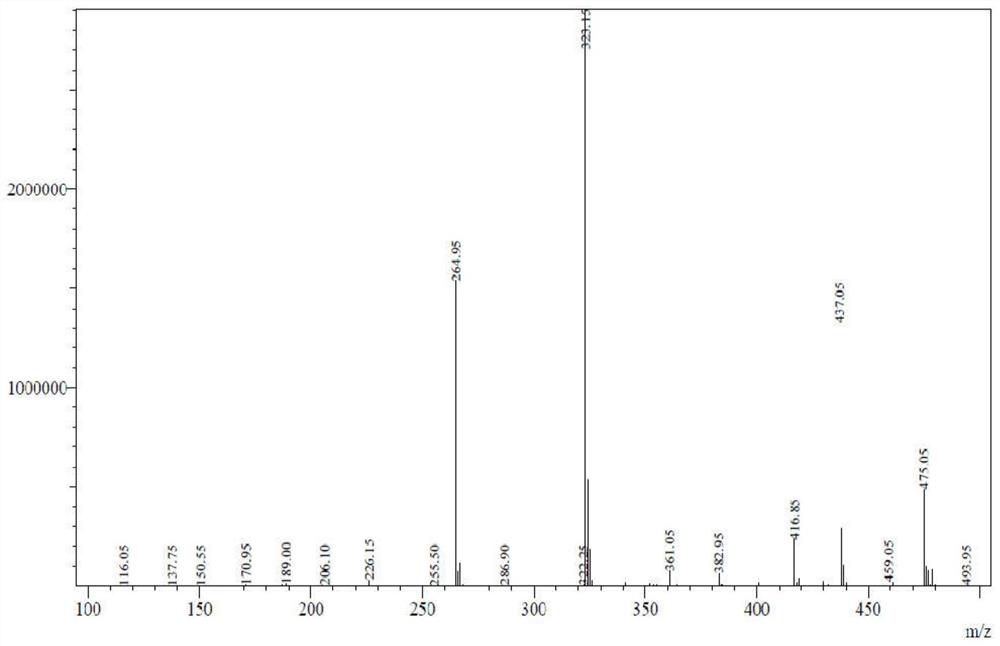
[0056] Add 440g of ethanol and 296g of water to a 1L four-neck flask, add trans-4-(methyl{7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidine-4 -yl}amino)cyclohexyl]methylsulfinate 73.9g (0.15mol), then add 52.3g (0.45mol) of ammonium sulfite, heat up to 75-85°C, and keep warm for 24-30 hours. Cool down to 20-25°C, add 47.1g (0.18mol) of tetrabutylammonium fluoride, keep the reaction at 25°C for 16 hours, adjust the pH value to 7-8 with phosphoric acid, cool down to 0-5°C, suction filter, filter The cake was dried at 80-85°C to obtain 42.1 g of trans-4-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclohexyl)methanesulfonic acid; yield 86.5% . HPLC purity 98.2%.
[0057] [M-1]=323.1 (see figure 2 )
Embodiment 3
[0059] Preparation of N-methyl-1-{trans-4-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclohexyl}methanesulfonamide:
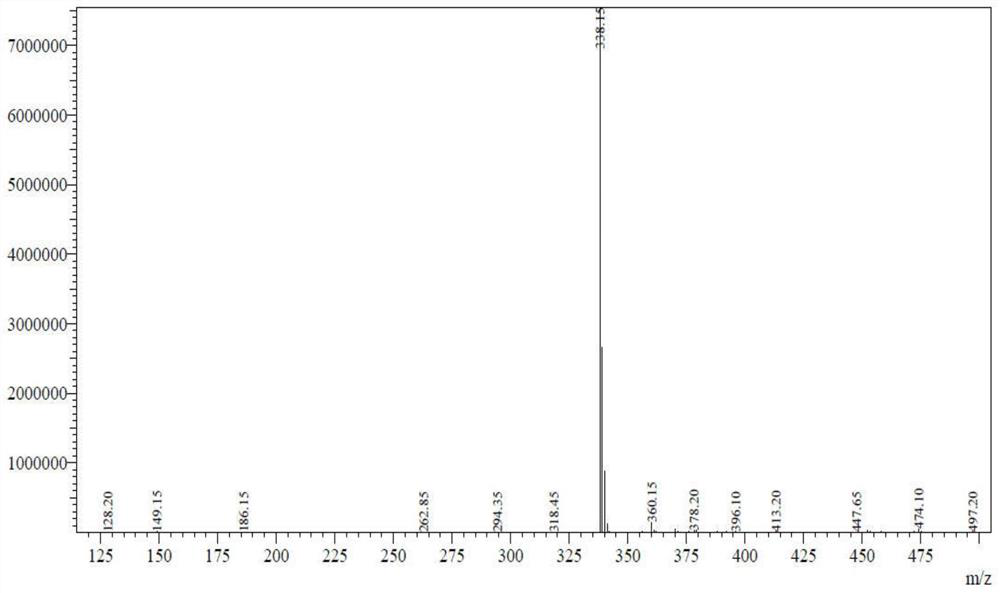
[0060] Add acetonitrile 130g to 1L four-neck flask, add trans-4-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclohexyl)methanesulfonic acid 32.4g (0.1mol ) and 3.5g (0.03mol) of tetramethylethylenediamine, lower the temperature to 5°C, add 15.3g (0.12mol) of oxalyl chloride dropwise, keep stirring at 5-10°C for at least 3 hours, and drop 35-40% methylamine aqueous solution 70g (0.9mol), control the temperature at 0-10°C, after the dropwise addition, keep stirring at 0-10°C for at least 0.5-1 hour, add 400g of drinking water, keep stirring for 1 hour, filter with suction, and dry the filter cake at 75-85°C Obtain 30.5 g of N-methyl-1-{trans-4-[methyl(7H-pyrrolo[23-d]pyrimidin-4-yl)amino]cyclohexyl}methanesulfonamide, yield 90.5%.HPLC 99.1% purity.
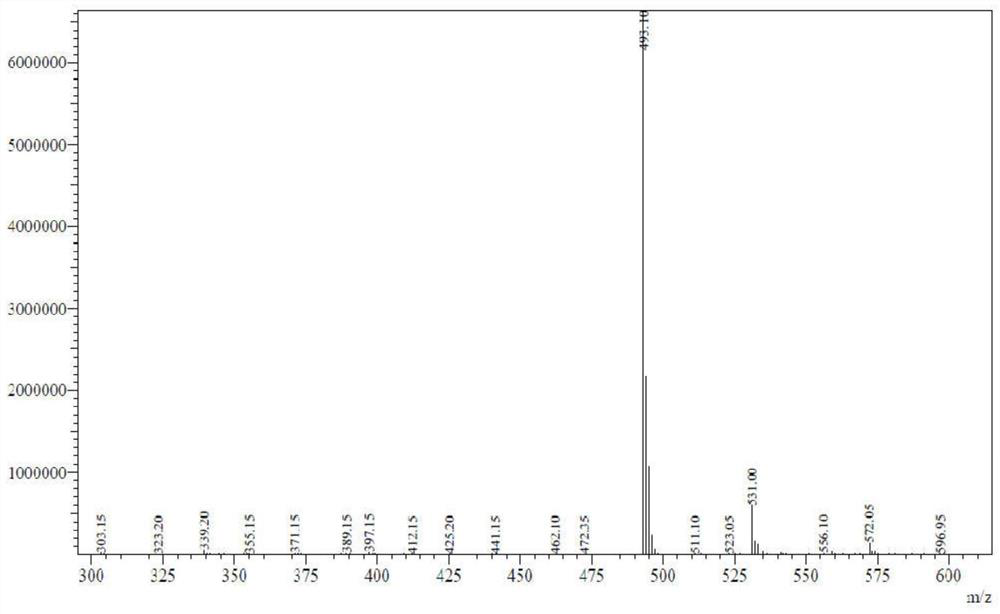
[0061] WNMR-I-500MHz nuclear magnetic resonance instrument nuclear magnetic hydrogen spectrum detection, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com