Application of malignant glioma biomarker
A malignant glioma and target technology, applied in the field of biotechnology, can solve the problems of no related reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
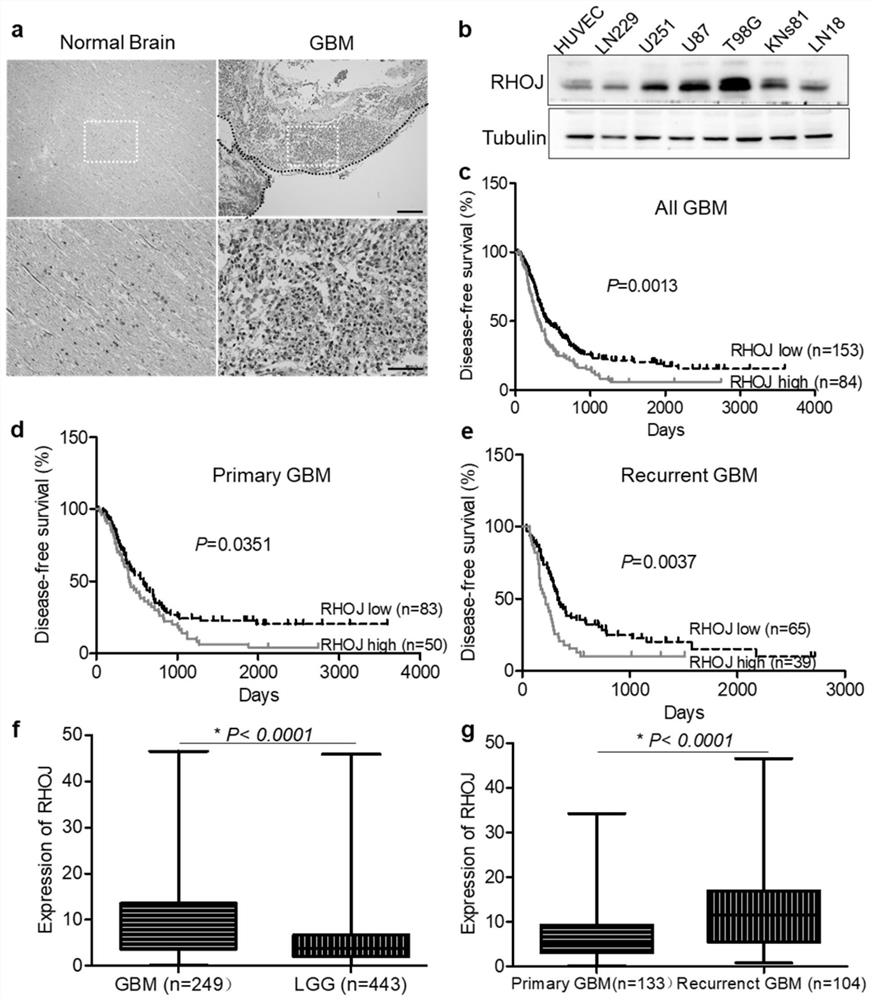
[0018] We performed RHOJ antibody (Sigma, HPA003050) immunohistochemical staining on the clinical tissue sections collected from patients with malignant glioma: put the collected pathological sections in xylene I for 5 minutes, replace them with xylene II for 5 minutes, and then soak them in absolute ethanol for 3 minutes , 95% ethanol for 3 minutes, 80% ethanol for 3 minutes, 70% ethanol for 3 minutes, tap water for 3 minutes for dewaxing and hydration, placed in antigen retrieval solution (citrate buffer, pH 6.0) and boiled at 95-98°C for 15 minutes, naturally Cool to room temperature for antigen retrieval, wash 3 times with PBST buffer, 3 min each time. Add goat serum blocking solution dropwise, room temperature for 30min. Shake off the blocking solution, add primary antibody (RHOJ, 1:400), overnight at 4°C. Wash 3 times with PBST buffer for 3 min each time, add secondary antibody dropwise, room temperature for 1-2 h, wash 3 times with PBST buffer for 3 min each time, deve...
Embodiment 2
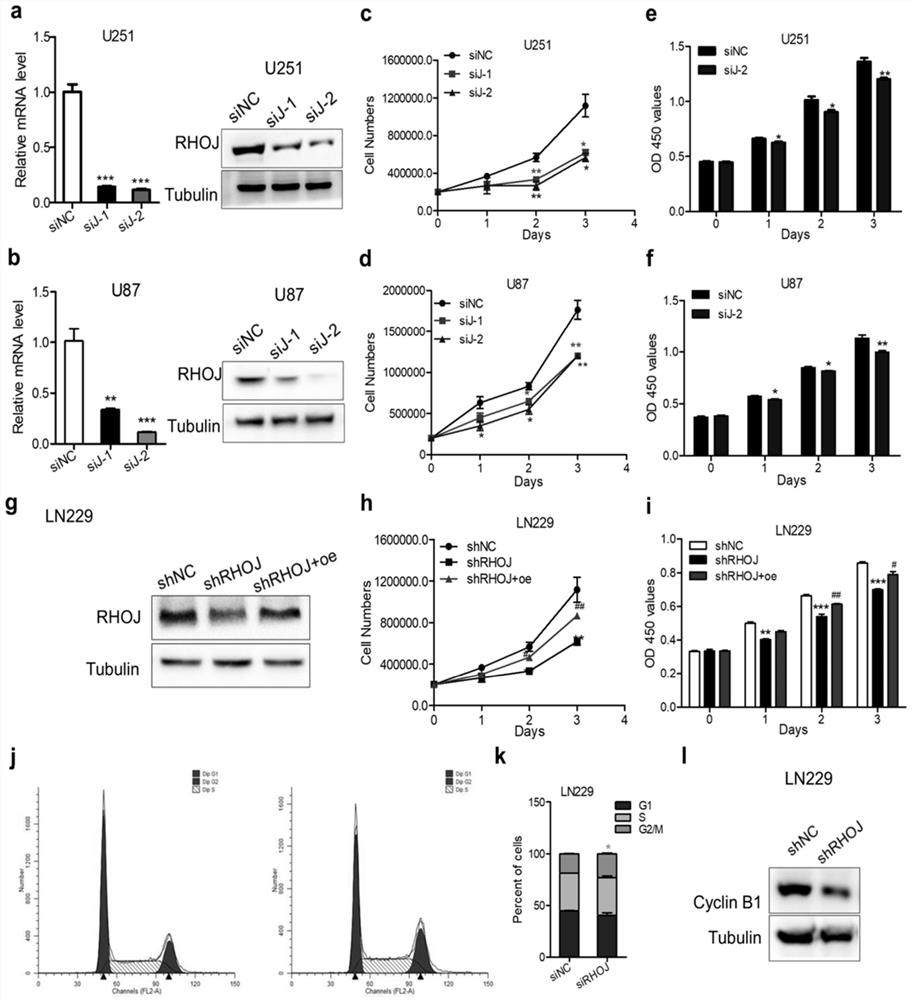
[0022] The siRNA that specifically inhibits RHOJ is based on the RHOJ sequence and the siRNA online design website provided by Invivogen (https: / / link.zhihu.com / ?target=http%3A / / www.invivogen.com / sirnawizard / siRNA.php) Two siRNAs were designed to prevent off-target effects. siRNA was synthesized and provided by Shanghai Gemma Pharmaceutical Technology Co., Ltd.
[0023] Construction of shRNA that specifically inhibits RHOJ and plasmids that overexpress RHOJ. The specific shRNA sequence was obtained from the official website of sigma company, and inserted into the PLKO.1 plasmid vector to construct the shRHOJ expression plasmid. The CDS region of the RHOJ gene was obtained from Pubmed, and primers were designed. The primers were synthesized and provided by GenScript Biotechnology Co., Ltd., and the full-length sequence of RHOJ was obtained by PCR cloning, and inserted into the eukaryotic expression vector plenti-CMV-GFP-puro. Transform the shRHOJ plasmid and the RHOJ expressi...
Embodiment 3
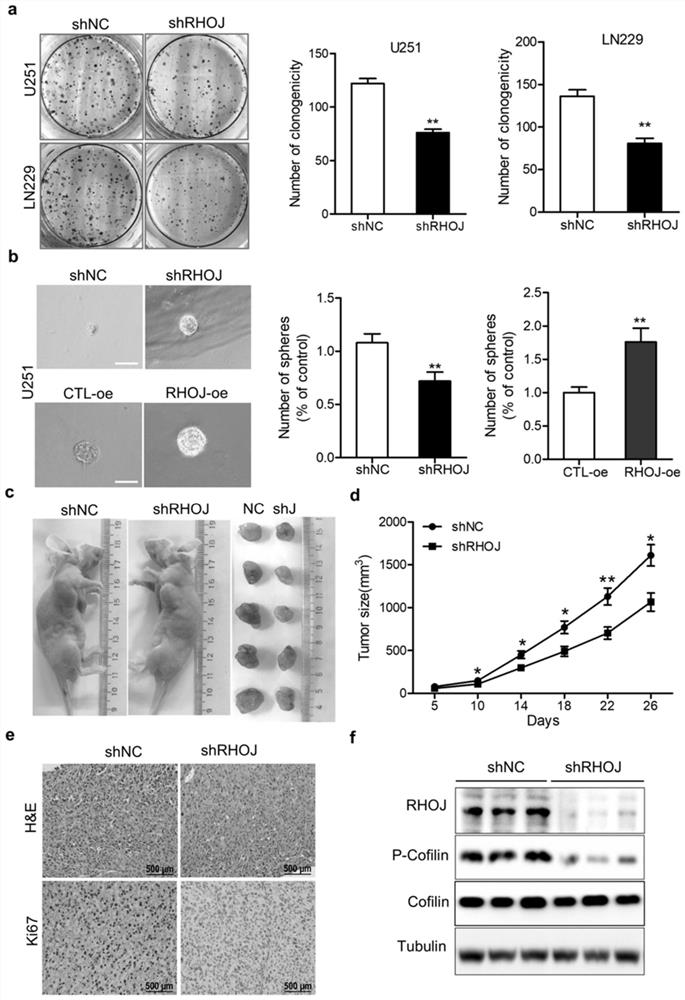
[0041] For Transwell migration assay, the cells were starved overnight in serum-free medium, then trypsinized, suspended in serum-free DMEM medium, and counted to adjust the concentration to 2×10 4 / mL; add 600 μL of DMEM complete medium containing 10% serum to the bottom of the 24-well plate, add 200 μL of cell suspension to the upper chamber, and then put it in the incubator for culture; 24 hours later, carefully take out the small chamber with tweezers, and suck off the upper chamber Liquid, add about 600 μL of 4% paraformaldehyde, fix at room temperature for 30 minutes; suck off the 4% paraformaldehyde in the upper chamber, add about 600 μL of crystal violet, and stain at room temperature for 30 minutes; wash gently with PBS several times, take out the small chamber, and suck out the upper chamber. The liquid in the upper chamber was carefully wiped off with a wet cotton swab; 5 fields of view were randomly selected under a 40x light microscope for observation, counting, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com