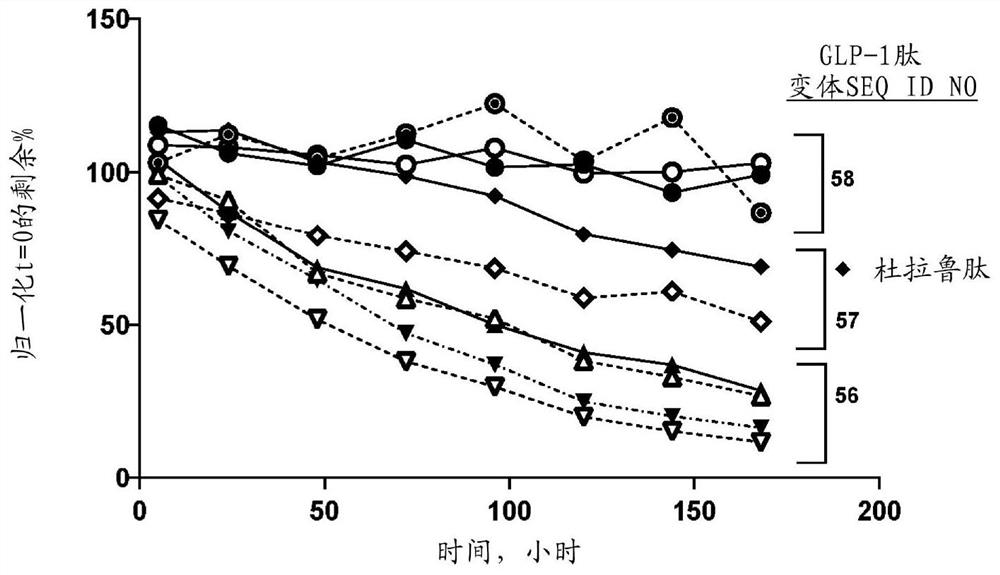
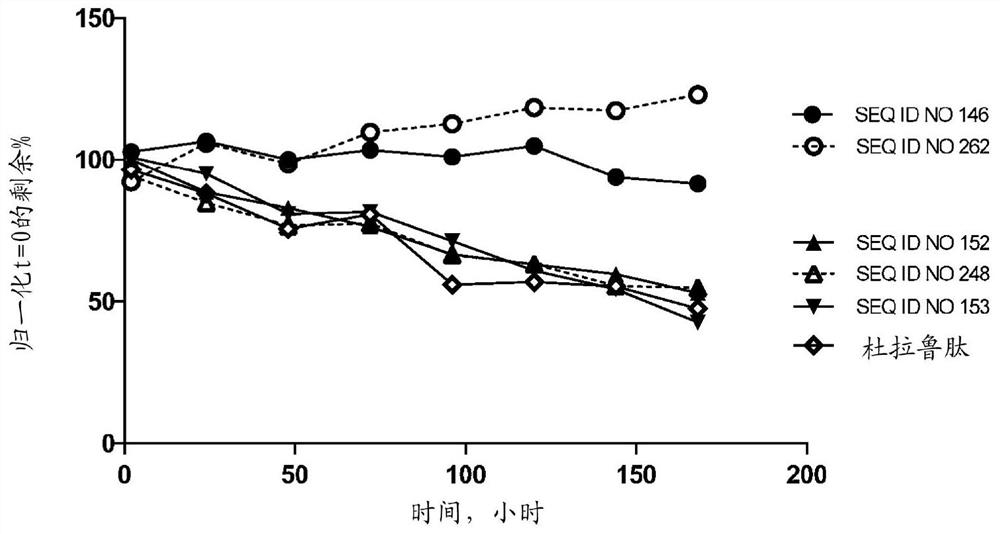
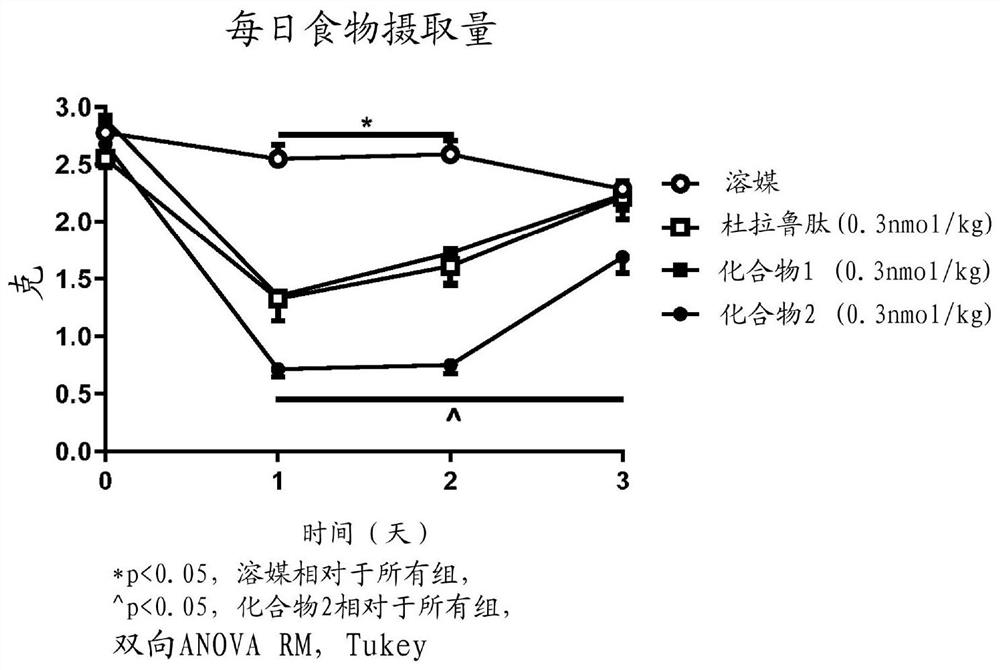
Glucagon like peptide 1 (glp-1) fusion peptide coupled cyclic peptide tyrosine tyrosine conjugates and uses thereof
A technology of GLP-1 and glucagon, applied in the field of cyclic peptide tyrosine-tyrosine conjugates, which can solve the problems of impracticality and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0357] Embodiment 1 is a conjugate comprising a glucagon-like peptide (GLP-1 ) fusion peptide coupled to a cyclic PYY peptide, wherein the GLP-1 fusion peptide comprises GLP-1 peptide, a first linker peptide, a hinge-Fc region peptide and a second linker peptide, wherein said first linker peptide is optionally absent.
Embodiment approach 2
[0358] Embodiment 2 is the conjugate according to embodiment 1, wherein the cyclic PYY peptide is represented by Formula I or a derivative or pharmaceutically acceptable salt thereof:
[0359]
[0360] in
[0361] p is 0 or 1;
[0362] m is 0, 1, 2, 3, 4 or 5;
[0363] n is 1, 2, 3 or 4;
[0364] q is 0 or 1; the precondition is only if Z 30 When not present, q is 1;
[0365] BRIDGE to -Ph-CH 2 -S-, -triazolyl-, -NHC(O)CH 2 S-, -SCH 2 C(O)NH-,
[0366] -(OCH 2 CH 2 ) 2 NHC(O)CH 2 S, -NHC(O)- or -CH 2 S-;
[0367] Z 4 is K, A, E, S or R;
[0368] Z 7 be A or K;
[0369] Z 9 is G or K;
[0370] Z 11 is D or K;
[0371] Z 22 be A or K;
[0372] Z 23 is S or K;
[0373] Z 26 be A or H;
[0374] Z 30 is L, W or absent,
[0375] The precondition is that only when q is 1, Z 30 does not exist;
[0376] Z 34 for
[0377] Z 35 for
[0378] Wherein said derivative is a compound of formula I modified by one or more methods selected from amidation,...
Embodiment approach 3
[0379] Embodiment 3 is the conjugate according to embodiment 2, wherein the cyclic PYY peptide is represented by Formula I or a derivative or pharmaceutically acceptable salt thereof, wherein:
[0380] p is 0 or 1;
[0381] m is 0, 1, 2, 3, 4 or 5;
[0382] n is 1, 2, 3 or 4;
[0383] q is 0 or 1; the precondition is only if Z 30 When not present, q is 1;
[0384] BRIDGE to -Ph-CH 2 -S-, -triazolyl-, -NHC(O)CH 2 S-, -SCH 2 C(O)NH 2 -,
[0385] -(OCH 2 CH 2 ) 2 NHC(O)CH 2 S, -NHC(O)- or -CH 2 S-;
[0386] Z 4 is K, A, E, S or R;
[0387] Z 7 is A or K, wherein the amino side chain of K is optionally substituted by
[0388] wherein i is an integer from 0 to 24, and X=Br, I or Cl,
[0389] -C(O)CH 2 Br, -C(O)CH 2 I or -C(O)CH 2 Cl;
[0390] Z 9 is G or K, wherein the amino side chain of K is optionally substituted by
[0391] wherein i is an integer from 0 to 24, and X=Br, I or Cl,
[0392] -C(O)CH 2 Br, -C(O)CH 2 I or -C(O)CH 2 Cl;
[0393] Z 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com