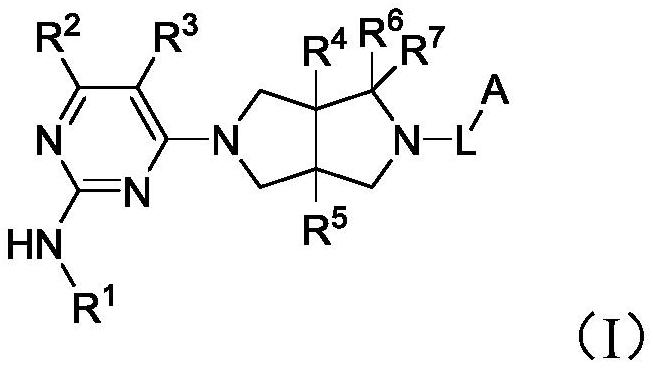
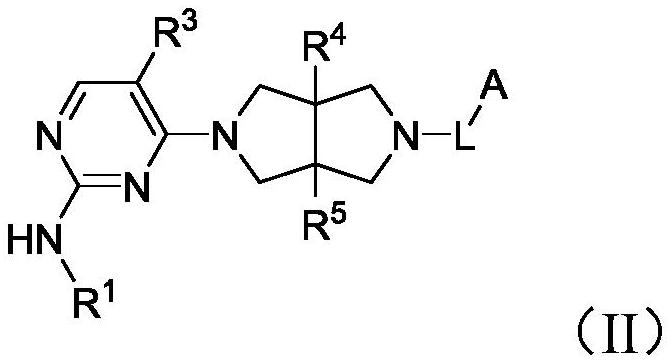
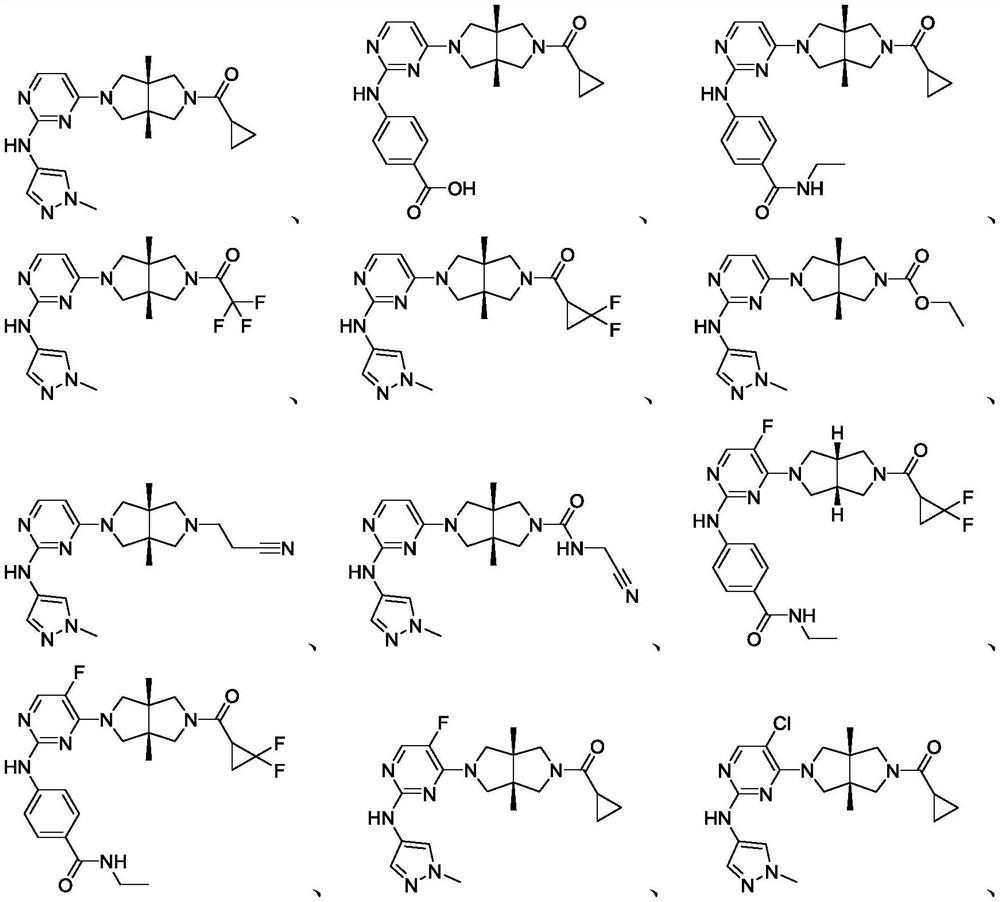
Heterocyclic compound as well as preparation method and medical application thereof
A technology of compound and heterocyclic group, applied in the field of heterocyclic compound, its preparation and its application in medicine, can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Cyclopropyl((3aR,6aS)-3a,6a-dimethyl-5-(2-((1-methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)hexahydropyrrole And[3,4-c]pyrrol-2(1H)-yl)methanone
[0134]
[0135] first step
[0136] 3,7-Dimethylethylene-1,5-xylenesulfonyl-1,5-diazacyclooctane
[0137] p-Toluenesulfonamide (17.12g, 100mmol), anhydrous potassium carbonate (27.6g, 200mmol) and anhydrous acetonitrile (200mL) were mixed, then 3-chloro-2-chloromethyl-1- Propylene 1a (12.5 g, 100 mmol) in acetonitrile (20 mL). After the mixture was stirred under reflux for 18 hours, it was cooled to room temperature, and water (250 mL) was added, followed by stirring for 30 minutes. The precipitate was collected by filtration to obtain 20 g of crude product. The crude product was purified by silica gel column chromatography (petroleum ether / dichloromethane=100 / 0 to 0 / 100) to obtain the target product (10.8 g, solid), yield: 48%.
[0138] MS m / z(ESI):447[M+1]
[0139] 1 H NMR (400MHz, CDCl 3 )δ7.70-7.64(m,4H),7.31(d,J...
Embodiment 2
[0165] 4-((4-((3aR,6aS)-5-(cyclopropylcarbonyl)-3a,6a-dimethylhexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)pyrimidine- 2-yl)amino)benzoic acid
[0166]
[0167] first step
[0168] (3aR,6aS)-5-(2-((4-(tert-butoxycarbonyl)phenyl)amino)pyrimidin-4-yl)-3a,6a-dimethylhexahydropyrrolo[3,4 -c]pyrrole-2(1H)-tert-butyl carboxylate
[0169] To a 10-mL microwave reaction vial, add (3aR,6aS)-5-(2-chloropyrimidin-4-yl)-3a,6a-dimethylhexahydropyrrolo[3,4-c]pyrrole-2- (1H)-tert-butylcarboxylate 1e (100mg, 0.283mmol), tert-butyl 4-aminobenzoate (55mg, 0.283mmol), p-toluenesulfonic acid (5mg, 0.0283mmol) and isopropanol (4mL ), then the mixture was stirred at 100 °C for 1 h in a microwave reactor. After cooling to room temperature, the solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=2 / 1 to 1 / 2) to obtain the target product (3aR, 6aS)-5-(2-( (4-(tert-butoxycarbonyl)phenyl)amino)pyrimidin-4-yl)-3a,6a-d...
Embodiment 3
[0181] 4-((4-((3aR,6aS)-5-(cyclopropylcarbonyl)-3a,6a-dimethylhexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)pyrimidine- 2-yl)amino)-N-ethylbenzamide
[0182]
[0183]4-((4-((3aR,6aS)-5-(cyclopropylcarbonyl)-3a,6a-dimethylhexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)pyrimidine -2-yl)amino)benzoic acid 2 (56mg, 0.133mmol) was dissolved in dichloromethane (10mL), then diisopropylethylamine (59mg, 0.153mmol) and 2-(7-oxybenzo Triazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (58 mg, 0.153 mmol). After stirring at room temperature for 5 minutes, 3 drops of aqueous ethylamine (65%-70%wt) were added. The mixture was stirred at room temperature for 30 minutes, then the solvent was removed under reduced pressure, and the residue was purified by reverse-phase preparative liquid chromatography to obtain the target product 4-((4-((3aR,6aS)-5-( Cyclopropylcarbonyl)-3a,6a-dimethylhexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)pyrimidin-2-yl)amino)-N-ethylbenzamide 3(3.3 mg, solid), yield: 6%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com