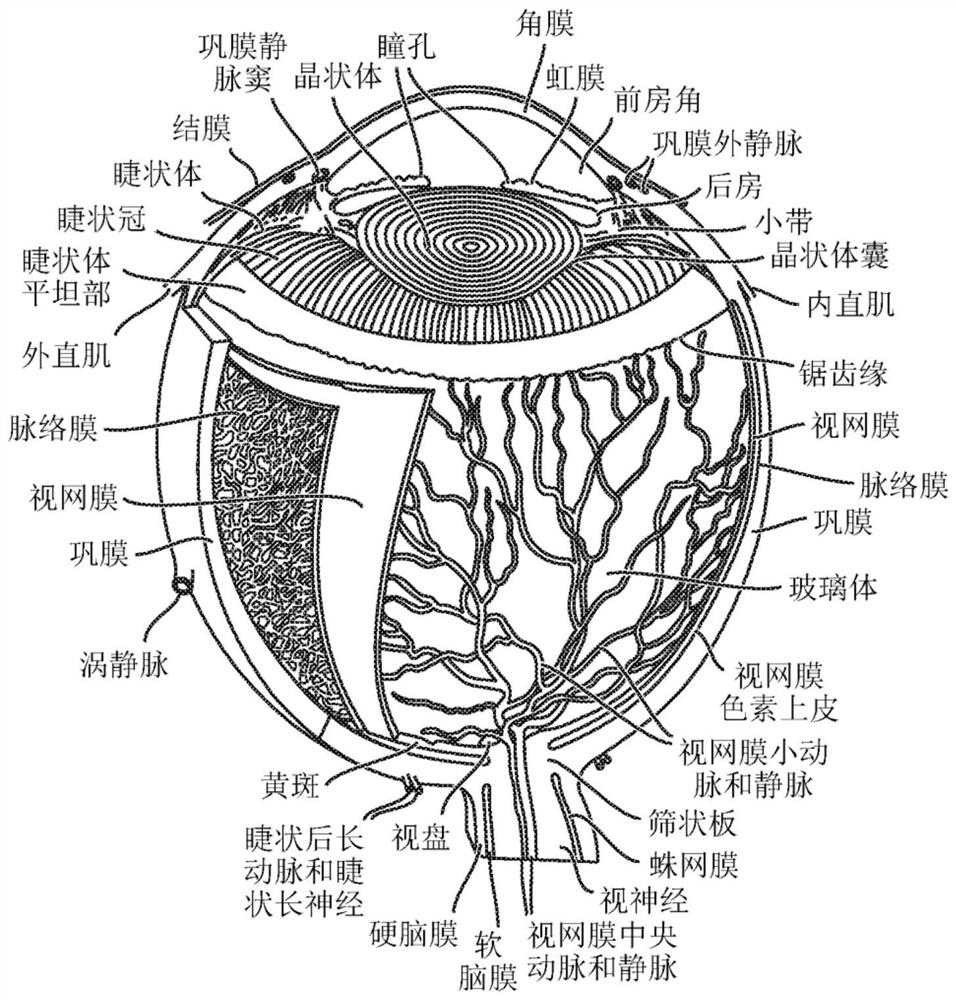
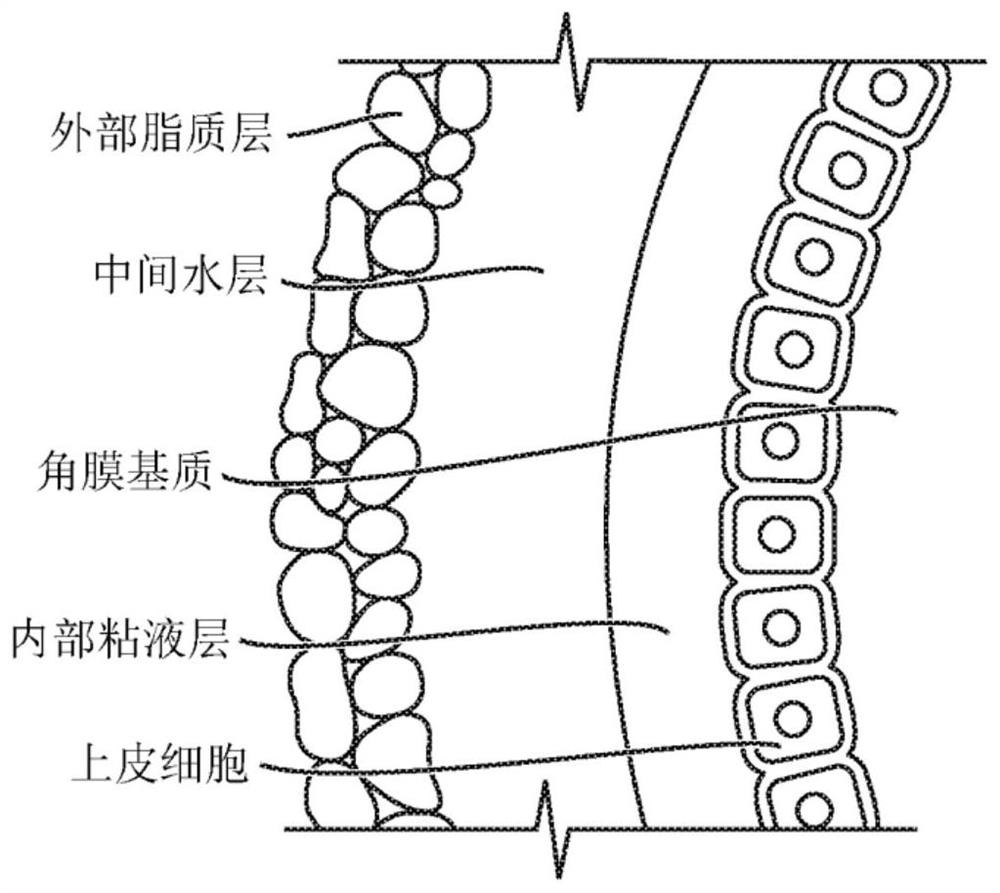
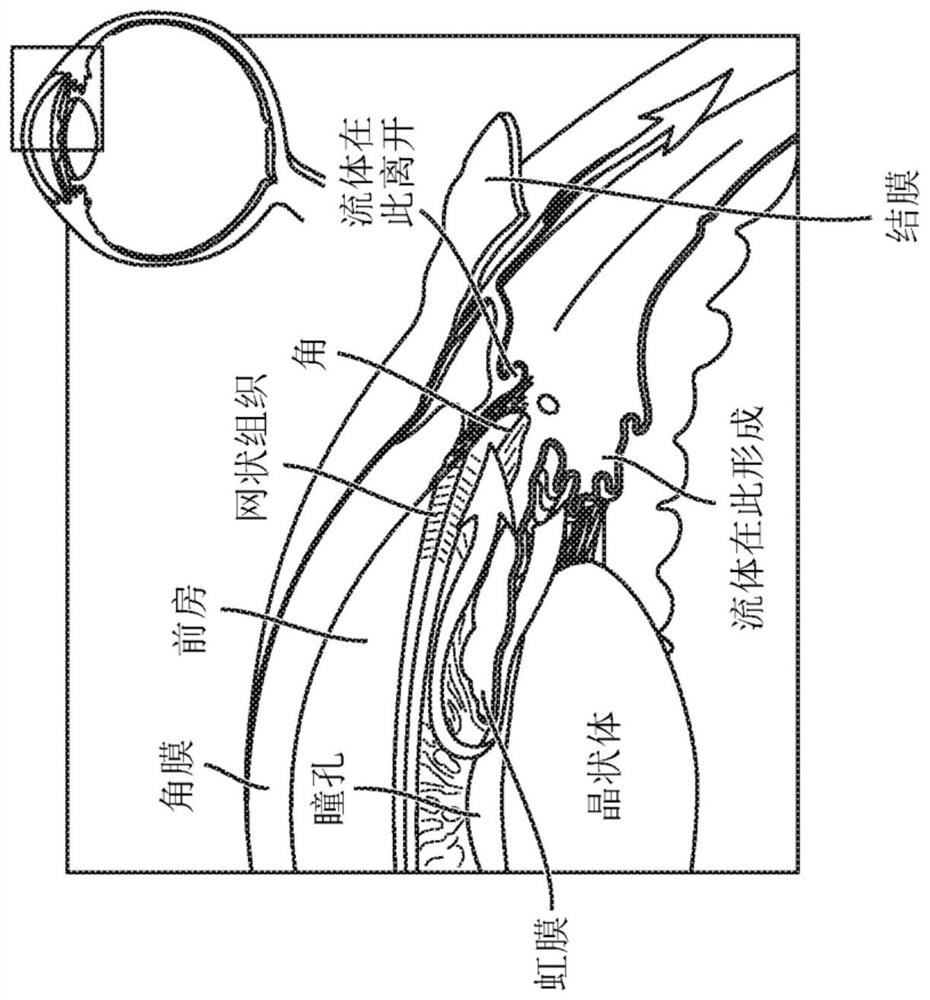
Sap and peptidomimetics for treatment of eye disease
An effective treatment and eye technology, applied in the fields of peptides, peptide sources, sensory diseases, etc., can solve the problems of not long-term use of corticosteroids, contraindications to patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0342] Example 1: SAP exhibits therapeutic effects in the eye
[0343] The anti-inflammatory effect of SAP was examined using a lipopolysaccharide ocular inflammation model, and the measurement of microglial activation was evaluated as an indicator of inflammation. Morphological changes in macrophages, such as microglia, can be used as markers of the presence and extent of inflammation. Methods for identifying and scoring microglial morphological changes in response to inflammatory stimuli are described in the art (see, e.g., Jonas et al., PLOS ONE 7(2):e30763 (2012) ).
[0344] Methods and Materials
[0345] animal
[0346] Adult white Sprague-Dawley rats weighing between 200 and 240 grams were housed on a 12 hr light / 12 hr dark cycle with free access to food and water. Animals were randomly divided into study or control groups.
[0347] intravitreal injection
[0348] Animals were anesthetized by intraperitoneal injection of 2% xylazine (5 mg / kg body weight) and 10% ...
example 2
[0368] Example 2: (RADA) 2 and EARA-based SAP exhibit therapeutic effects on the eye
[0369] Methods and Materials
[0370] animal
[0371] Adult White Sprague-Dawley rats weighing between 200 and 240 grams were housed on a 12 hr light / 12 hr dark cycle with free access to food and water. Animals were randomly divided into study or control groups.
[0372] processing and analysis
[0373] Intravitreal injections, immunohistochemical staining and assessment of intraocular inflammation were generally performed as described in Example 1.
[0374] Administered by direct intravitreal injection alone or with 0.1%, 1.0% and 10.0% (RADA) 2 (SEQ ID NO: 88) solution combined with 1 μl of sterile lipopolysaccharide (LPS; from Salmonella typhimurium, catalog number L-7261; Sigma-Aldrich, St. Louis, MO). Animals were sacrificed 24 hours after treatment. Repeat the experiment at least 5 times.
[0375] In some experiments, as above for (RADA) 2 (SEQ ID NO:88), the evaluation cont...
example 3
[0382] Example 3: Delivery of SAP to the retina by application of eye drops
[0383] The fate of SAP when applied to the eye was investigated. Specifically, the ability of SAP to reach the retina by applying the SAP eye drop solution to the cornea was evaluated using the SAP-Cy5 conjugate.
[0384] method
[0385] animal
[0386] Adult White Sprague-Dawley rats weighing between 200 and 240 grams were housed on a 12 hr light / 12 hr dark cycle with free access to food and water. Animals were randomly divided into experimental or control groups.
[0387] Conjugation of Cy5.5 to RADA
[0388] Cy5.5 and RADA (SEQ ID NO:57), Cy5.5 and RADARADA ((RADA) 2 ; SEQ ID NO:88) and Cy5.5 and H 2 N(RADA) 4 CONH 2 The conjugate of (SEQ ID NO:433) was prepared according to a modified protocol of Chen X. et al., Cancer Res., 64(21):8009-8014 (2004).
[0389] In one experiment, the RADA-4-Cy5.5 conjugate was prepared as follows: 0.5 mg of H 2 N(RADA) 4 CONH 2 Dissolve in 50 μL of DMS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com