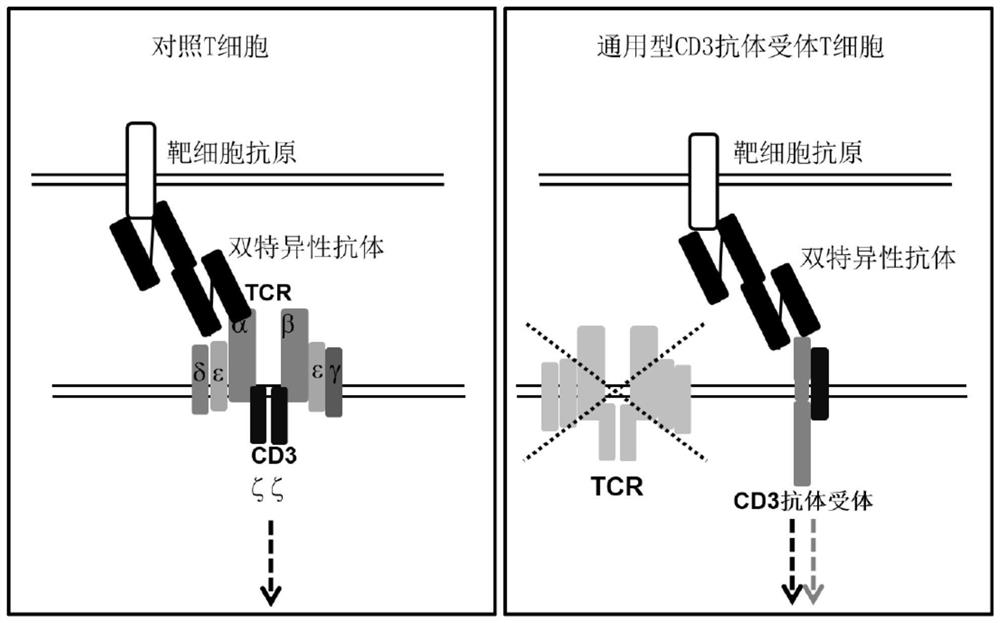
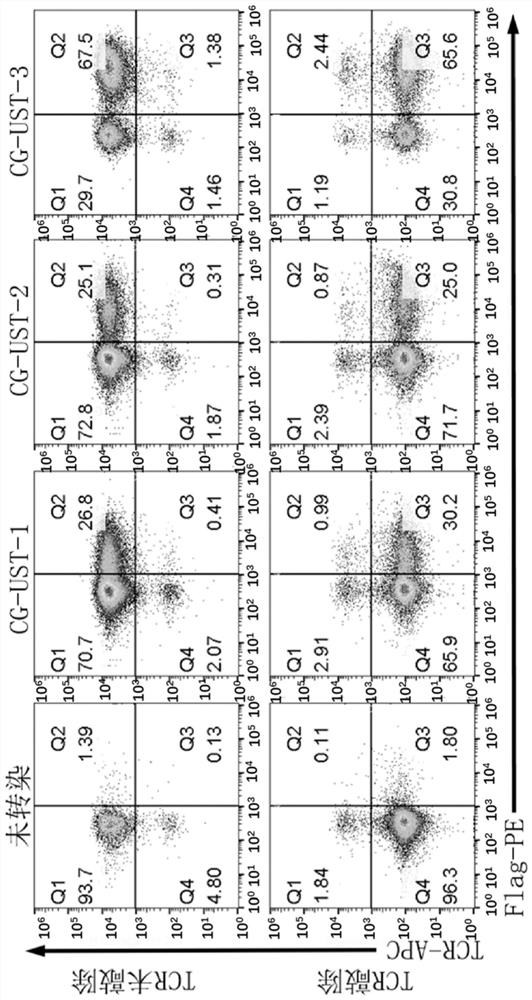
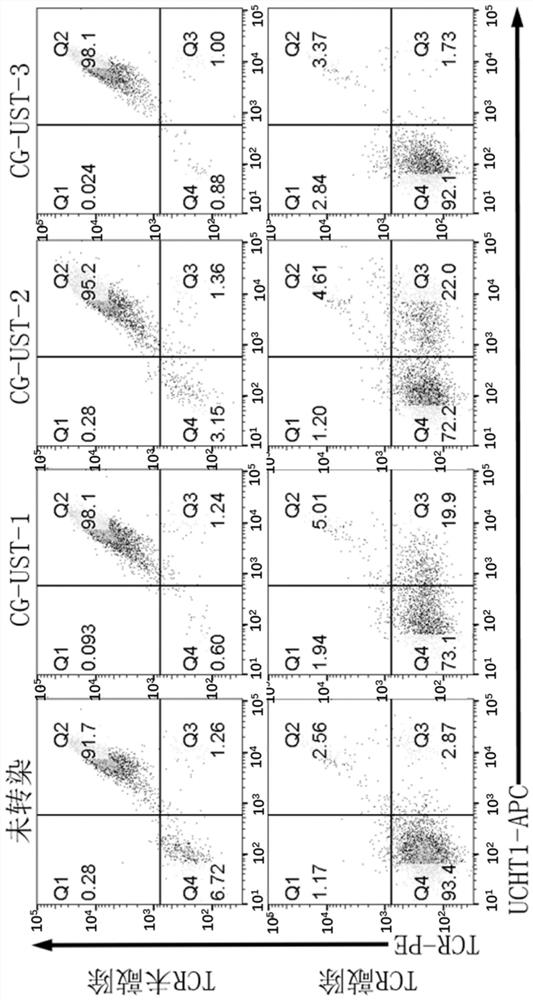
Immune cell for expressing CD3 antibody receptor complex and application thereof
A technology of immune cells and complexes, applied in the field of immune cells, which can solve problems such as cytotoxicity, B cell and immunoglobulin deficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0242] Example 1 Design of CD3 Antibody Receptor Complex Molecules and Construction of Plasmids
[0243] (1) Design molecules
[0244]The gene sequence information (Table 1) was searched from the NCBI website database (https: / / www.ncbi.nlm.nih.gov / ), and the gene CG-UST-1 (SEQ ID NO. 13), CG-UST-1 comprises two parts, is the tandem of two CD3 recombinant protein genes, and the first CD3 recombinant protein comprises CD3 epsilon ectodomain, CD28 transmembrane region, CD28 intracellular domain and CD3 zeta intracellular domain (SEQ ID NO:10). The second CD3 recombinant protein comprises CD3 gamma extracellular domain, CD28 transmembrane region and CD3 gamma intracellular domain (SEQ ID NO: 11). The two CD3 recombinant proteins are connected through the linker T2A gene (SEQ ID NO.16).
[0245] The CD3 gamma intracellular domain of the gene encoding the second CD3 recombinant protein of CG-UST-1 was replaced with a gene encoding a short peptide to obtain CG-UST-2 (SEQ ID NO.14)...
Embodiment 2
[0248] Example 2 Preparation of lentivirus
[0249] (1) Extract plasmid
[0250] The lentiviral vector plasmid constructed above was re-transformed into Escherichia coli. Pick a single clone from the transformed plate and put it into a 3ml shaking tube of liquid LB medium containing ampicillin, rotate at 220rpm, and culture it on a shaking table for 8h; draw 500μl from the activated bacterial solution and inoculate it into 250ml containing ammonia Bian penicillin liquid LB culture medium, 220rpm, shaker shake culture 12-16h. Use the Qiagen HiSpeed Plasmid Maxi Kit kit (Catalog No.: 12662) for plasmid extraction according to the experimental procedure provided by the kit. After extracting the plasmid, Nanodrop (Thermo Fisher Scientific) was used to detect the plasmid concentration and the supercoiled plasmid content was detected by DNA agarose gel.
[0251] (2) Culture 293T cells
[0252] After the frozen 293T cells (ATCC) were taken out from the liquid nitrogen, they wer...
Embodiment 3
[0257] Example 3 Preparation of general-purpose T cells expressing CD3 antibody-receptor complex
[0258] PMBCs from peripheral blood of healthy donors (purchased from Miaotong Biotech) were isolated using an apheresis machine. Dilute PBMCs to 2×106. T cells were activated using CD3 / CD28 magnetic beads (Thermo Fisher Scientific) at a cell to bead ratio of 1:3 with the addition of IL-2 (PeproTech; 200-02). On the 3rd day after activation, the concentrated lentivirus was added to the T cell culture flask to transfect T cells. On day 5 after T cell activation, TCR and B2M in T cells were knocked out using CRISPR / Cas9 to construct universal T cells. The used gRNA sequence and operation process refer to Example 3 of patent WO2019 / 011118.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com