A two-photon probe based on fluorescence energy resonance transfer mechanism and its application
An energy resonance transfer and two-photon technology, applied in the field of chemical detection, can solve problems such as threats, needs to be improved, and phosgene leakage to public health, and achieve the effects of large Stokes shift, superior selectivity, and excellent emission signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
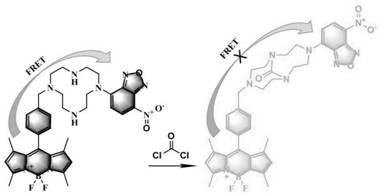
[0046] A Two-Photon Probe Based on Fluorescence Energy Resonance Transfer As a fluorescence donor, the 7-nitrobenzo-2-oxa-1,3-oxadiazole group acts as an acceptor, with two secondary amines with strong nucleophilicity as the response sites, and its chemical name is, 5 -Fluoro-1,3,7,9-tetramethyl-10-(4-(7-(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)-1 ,4,7,10-tetraazacyclododecyldodecyl-1-yl)phenyl)-5H-dipyrrolo[1,2-c:2',1'-f][1 ,3,2] Diazaboron-4-fluoride, the chemical structure of which is,
[0047]
[0048] The present invention also includes the application of a two-photon probe based on the fluorescence energy resonance transfer mechanism, using absorbent cotton as the carrier of the two-photon probe, and realizing the gaseous state above or below the safe threshold concentration in the single-photon and two-photon modes respectively. Phosgene detection, fluorescence imaging and confocal imaging.
[0049] The reaction mechanism of described detection is:
[0050] With 4,4...
Embodiment 2
[0052] Study on the Fluorescence Spectral Properties of the Probe's Responsiveness to Phosgene
[0053] (1) Prepare a chloroform solution with a probe concentration of 10 mM, prepare a triphosgene concentration of 10 mM, and contain a chloroform solution with a mole fraction of 5% triethylamine, which is a solution with a phosgene concentration of 30 mM;
[0054] (2) First add 10 μL of probe solution to a 5 mL Ep tube, add 2 mL of chloroform solution, and take 0 μL, 1 μL, 2 μL, 3 μL, 4 μL, 5 μL, 6 μL, 7 μL, 8 μL, 9 μL and 10 μL of the solution at a concentration of 10 mM A total of 11 parts of the triphosgene solution were added to the Ep tubes respectively.
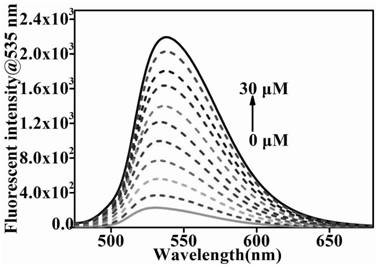
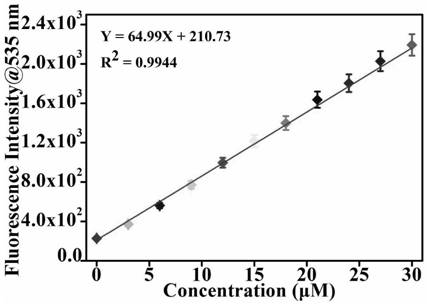
[0055] (3) After reacting for 1min, test the fluorescence intensity by a fluorescence spectrophotometer to obtain the fluorescence intensity, and the excitation wavelength for detecting the fluorescence intensity of phosgene is 455nm; obtain the fluorescence titration spectrum of the probe, see figure 2 . It can be se...
Embodiment 3
[0058] Study on the Spectral Properties of the Probe's Selectivity to Phosgene
[0059] (1) Prepare DCP, DECP, DNCP, POC, (COCl) at a concentration of 10mM 2 ,BsCl,p-TsCl,CH 3 COCl, POCl 3 , triphosgene and TEA solution, the solvent is chloroform.
[0060] (2) First add 10 μL, 10 mM probe solution to a 5 mL Ep tube, add 2 mL of chloroform solution, and mix well. Take 60 μL of the above-mentioned prepared solutions and add them to the Ep tubes respectively.
[0061] (3) After reacting for 5 minutes, test the fluorescence intensity by a fluorescence spectrophotometer to obtain the fluorescence intensity ratio, and the excitation wavelength for detecting the fluorescence intensity of phosgene is 455nm;
[0062] (4) Take the substance type as the abscissa and the fluorescence intensity ratio as the ordinate to obtain a histogram about the selectivity of the probe to phosgene, see Figure 4 .
[0063] The addition of phosgene in the figure triggers obvious fluorescence enhanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com