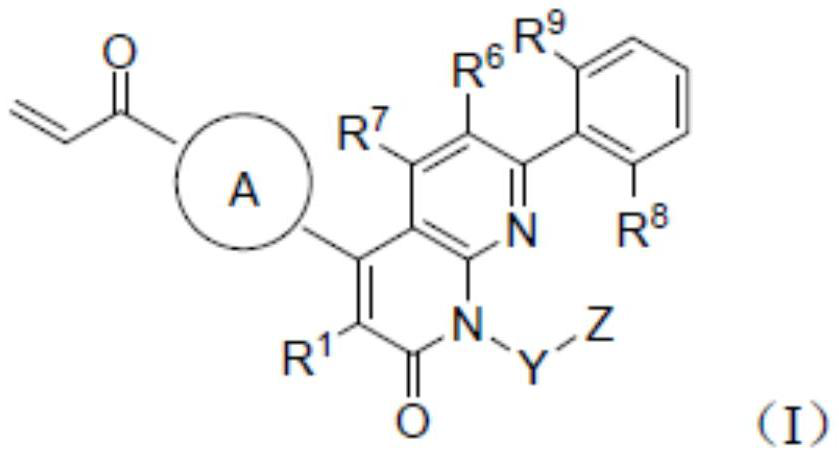
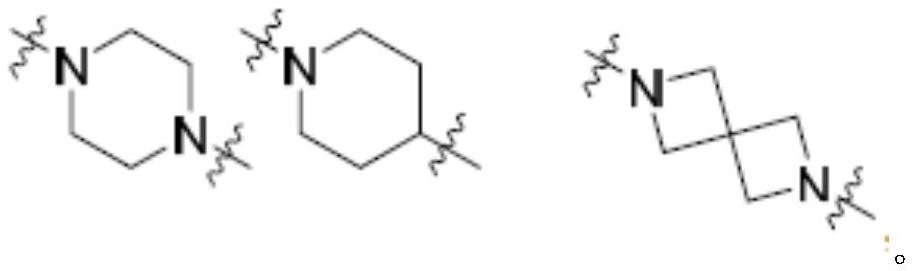
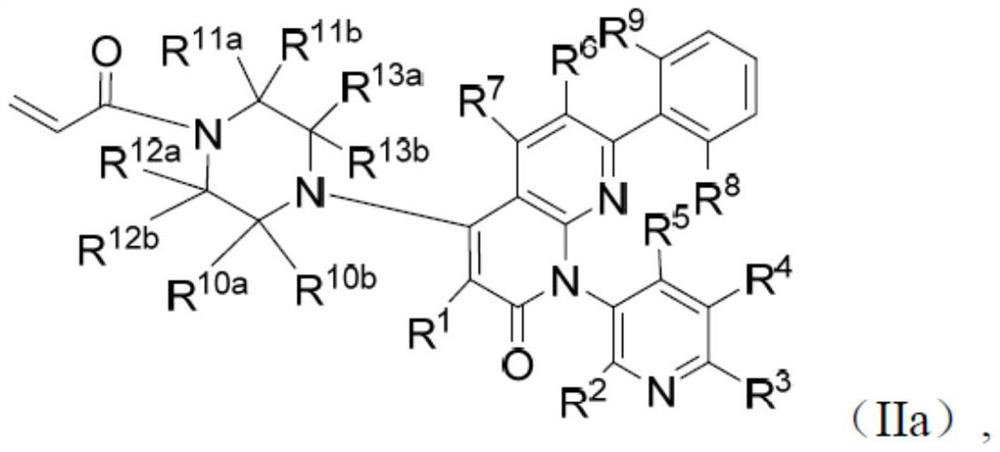
KRAS G12C inhibitor and application thereof
A use and compound technology, applied in the field of KRASG12C inhibitors, can solve the problems of lack of multidrug resistance, reducing the killing effect of anti-tumor drugs on tumors, reducing the sensitivity of tumors to anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0121]Compound preparation method
[0122]The compounds disclosed herein can be synthesized by many specific methods. Summarizing examples of specific synthetic routes and the following general schemes are intended to provide guidance for ordinary skilled synthetic chemists. Those skilled in the art will easily understand the solvent, concentration, reagents, protecting groups, sequence of synthetic steps, time, temperature, etc. Make modifications as needed within the scope of the skill and judgment of ordinary technicians.
[0123]The compound of formula (IIa) as disclosed herein can be synthesized as described in Method 1:
[0124]
[0125]The compound of formula (IIb) as disclosed herein can be synthesized as described in Method 2:
[0126]
[0127]The compound of formula (IIc) as disclosed herein can be synthesized as described in Method 3:
[0128]
[0129]use
[0130]The present invention also provides a use of the compound of the present invention to prepare a pharmaceutical composition for: (i) preve...
Embodiment
[0180]The present invention can be better understood according to the following examples. However, for those skilled in the art, it is easy to understand that the contents described in the embodiments are only intended to illustrate the present invention, and not to limit the present invention described in detail in the claims.
[0181]In this example, the compound AMG510 is prepared by referring to the method of WO2018217651A1:
[0182]
[0183]Example 1 Preparation of compound of formula I1
[0184]Compound of formula I1
[0185]4-(4-acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methyl Pyridin-3-yl)-1,8-naphthyridin-2(1H)-one (I1 compound)
[0186]
[0187]Step 1: Preparation of compound I-03
[0188]
[0189]Under nitrogen, to compound I-01 (10.0g, 52.36mmol) in 1,4-dioxane / H2Compound I-02 (13.3g, 78.53mmol) was added to the solution in O (100 / 10mL), Na2CO3(16.6 g, 157.08 mmol) and Pd(PPh3)4 (6.1 g, 5.24 mmol), the reaction mixture was heated to 110°C and stirred o...
Embodiment 5
[0315]Example 5: Compound of formula I5
[0316]Compound of formula I5
[0317]4-(4-acryloyl-2-methylpiperazin-1-yl)-3,6-difluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl- 4-methylpyridin-3-yl)-1,8-naphthyridin-2(1H)-one
[0318]
[0319]Step 1: Synthesis of I5-03
[0320]
[0321]To the dioxane / H2O (100mL / 10mL) solution of compound I5-01 (10g, 52.36mmol) was added compound I5-02 (10.68g, 62.83mmol), Na2CO3(16.6 g, 157.08 mmol) and Pd(pph3)4 (6.1 g, 5.24 mmol). The resulting mixture was stirred at 110°C overnight.
[0322]Use H2The reaction solution was diluted with O (100 mL), and extracted with EA (200 mL×2). The combined organic layer was washed with brine (100 mL×2), and washed with Na2SO4Dry, filter and concentrate. The residue was purified by silica gel chromatography (PE / EA=10 / 1) to obtain compound I5-03 (4.96 g, 34% yield) as a yellow solid.
[0323]MS Calcd.:280; MS Found:281[M+H]+.
[0324]Step 2: Synthesis of I5-05
[0325]
[0326]To a solution of compound I5-03 (2g, 7.13mmol) in toluene (30mL) was a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com