Car-cd30 t cells for treatment of cd30+ tumors
A 1. CD30, cell technology, applied in the field of CAR-CD30 T cells, can solve the problem that the clinical benefit is not the best
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0226] Example 1: In vitro and in vivo design and research of CAR-CD30 according to the present invention
[0227] Materials and methods
[0228] Design of CAR-CD30 plasmid (construct)
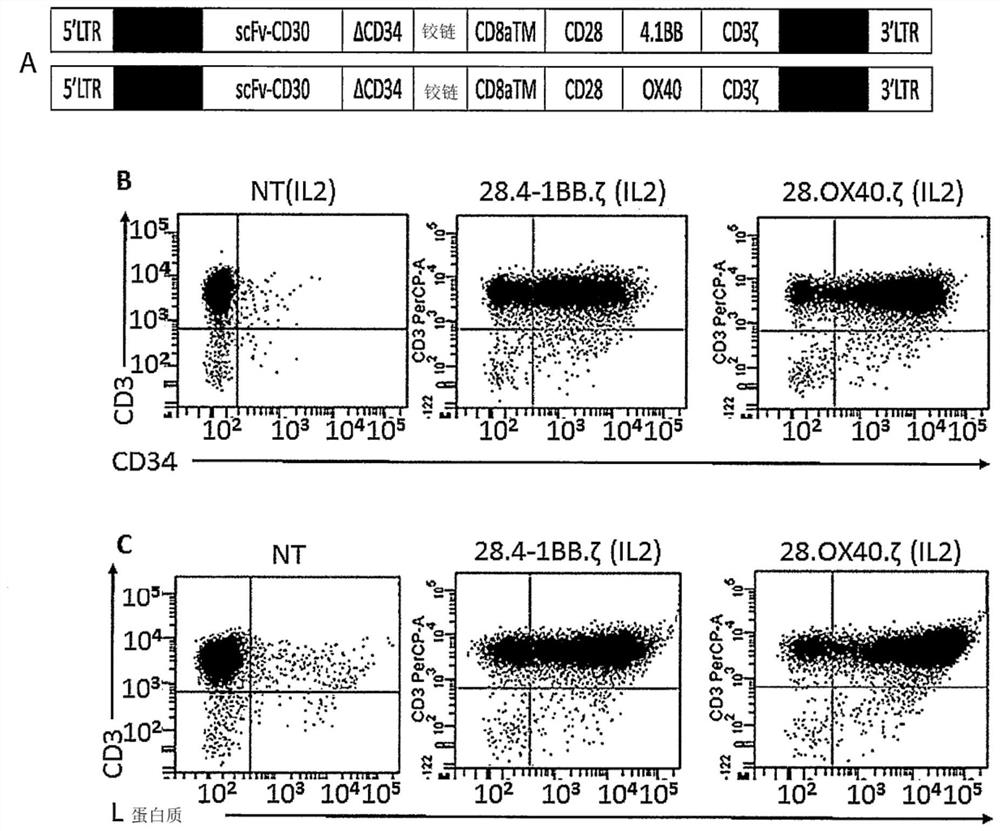
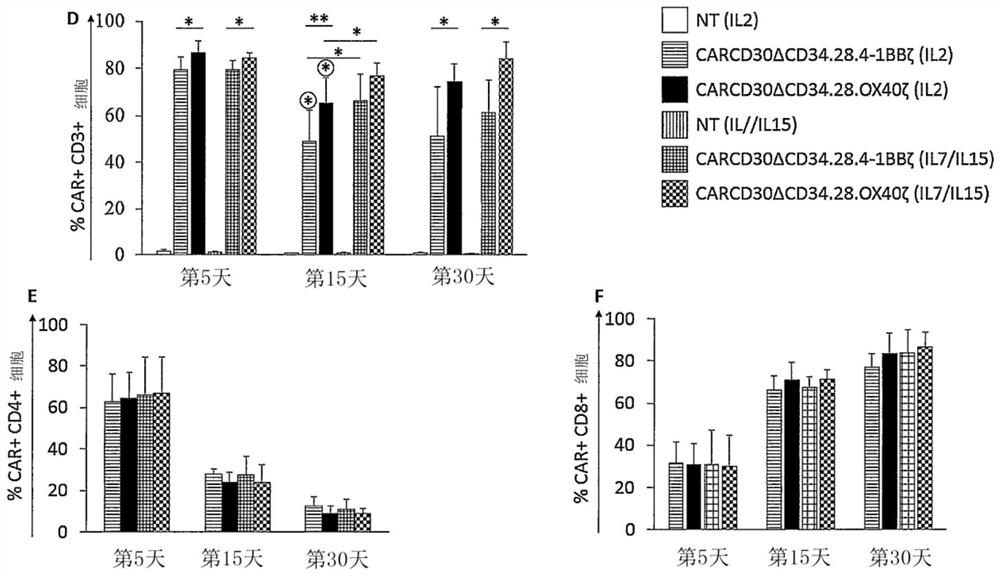
[0229] Two clinical-grade "third" generation retroviral vectors, SFG, have been designed carrying an anti-CD30 single-chain variable fragment (scFv) derived from an IgG(AC10)-like murine antibody, codon-optimized human CD8 hinge - the expression cassette of the codon-optimized signaling domain of the transmembrane domain linked to the two co-stimulatory domains CD28, 4-1BB (CD137) or OX40 and CD3-zeta ( figure 1 A). The CD30-specific single-chain variable fragment (scFv) is a fusion protein of 111 amino acids (aa) of the variable region of the immunoglobulin light chain (VL), consisting of an 8-amino acid flexible region (27) (short linker peptide ) is linked to 117 aa of the immunoglobulin heavy chain (VH). In particular, the scFv AC10 was cloned in the same reading frame as a codon-opti...
Embodiment 2
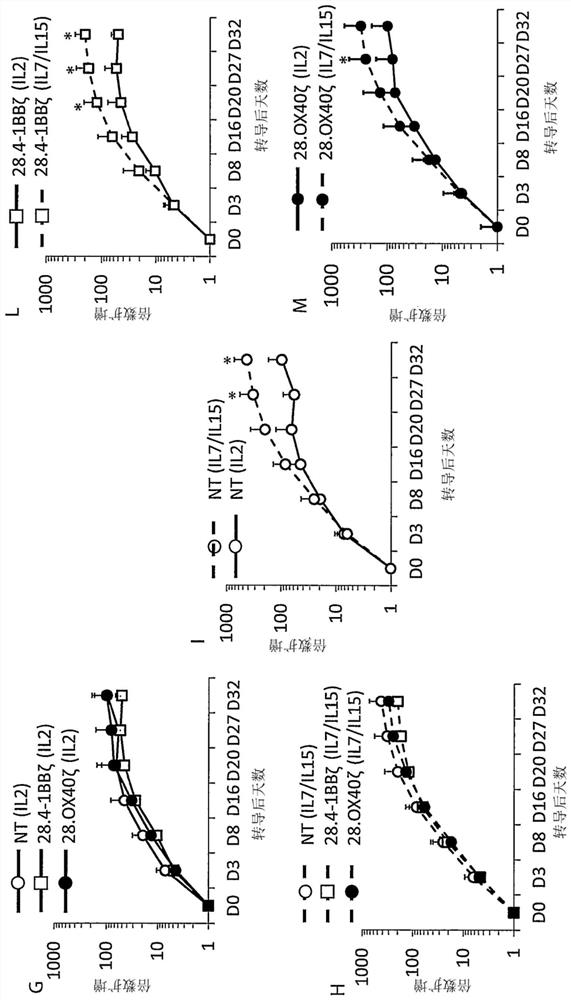
[0296] Example 2: Tumor regulation of memory and exhaustion in CAR.CD30 T cells (28.OX40.ζ T cells and 28.4-1BB.ζ T cells) according to the invention
[0297] Materials and methods
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com