Quinazoline hydroxamic acid derivative and preparation method and application thereof
A technology of quinazoline hydroxamic acid and derivatives is applied in the field of medicine and achieves the effects of good application prospect, simple preparation method and good anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
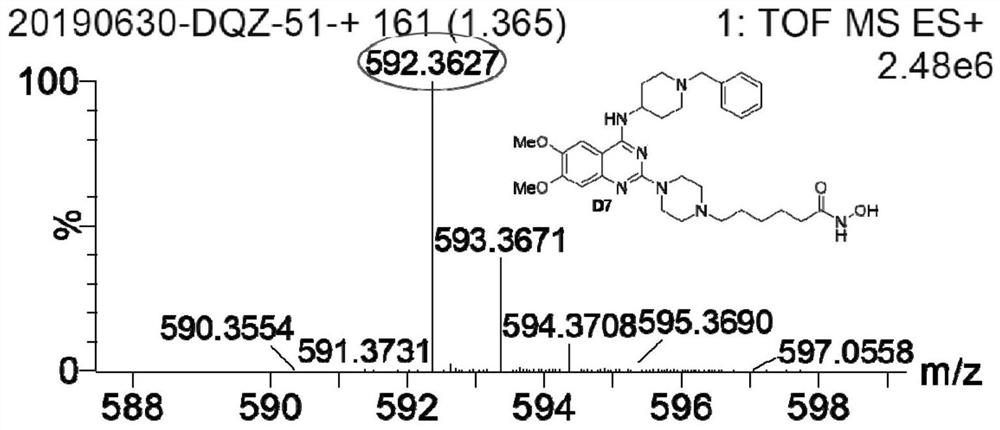
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 compound D1
Embodiment 1A
[0055] Example 1A intermediate 2-chloro-6,7-dimethoxy-N-(1-methylpiperidin-4-yl)quinazolin-4-amine (compound shown in formula III)
[0056] With 2,4-dichloro-6,7-dimethoxyquinazoline (compound shown in formula II) (1eq) and corresponding carrying R 1 The amino derivative of the group (2eq) was added to a round bottom flask, dissolved in an organic solvent such as tetrahydrofuran, stirred at room temperature under nitrogen protection, and reacted overnight, and then extracted with dichloromethane to obtain a crude product, which was separated by column chromatography to obtain a pure product.
Embodiment 1B
[0057] Example 1B 6-(6,7-dimethoxy-4-((1-methylpiperidine) quinazoline-amino)) methyl hexanoate
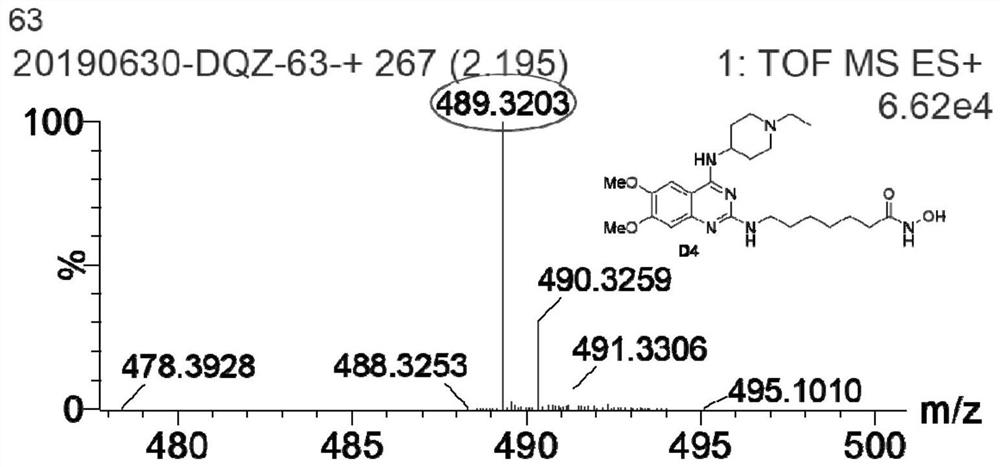
[0058] (compound shown in formula IV, Y=2-aminocaproamide, R 1 =1-methylpiperidine)
[0059] This step reaction is completed by nucleophilic substitution reaction, and the general process is as follows: the compound (1eq) obtained in Example 1A is dissolved in an organic solution such as acetonitrile, and DIEA (2eq) and the corresponding fatty acid ester of terminal primary amine (6-amino Methyl hexanoate) (2eq), react under nitrogen protection at 80°C for 2 hours, and spot plate monitoring until the reaction of the compound of Example 1A is complete. Add water and dichloromethane for extraction, and the organic phase is separated by column chromatography to obtain the product.
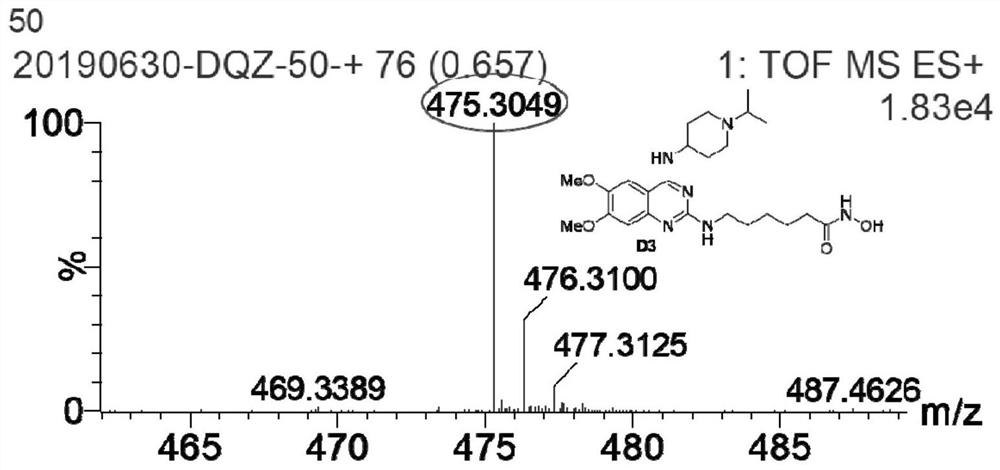
[0060] Example 1C 6-((6,7-dimethoxy-4-((1-methylpiperidin-4-yl) amino) quinazoline-2-yl) amino)-N-hydroxyl caproamide ( D1)
[0061] (compound shown in formula I, Y=2-aminocaproamide, R 1 =1-methylpi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap