Clinical trial subject recruitment system
A technology for clinical trials and subjects, applied in the medical field, can solve problems such as low recruitment efficiency, achieve the effects of improving the success rate of recruitment, reducing workload, and increasing the degree of diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
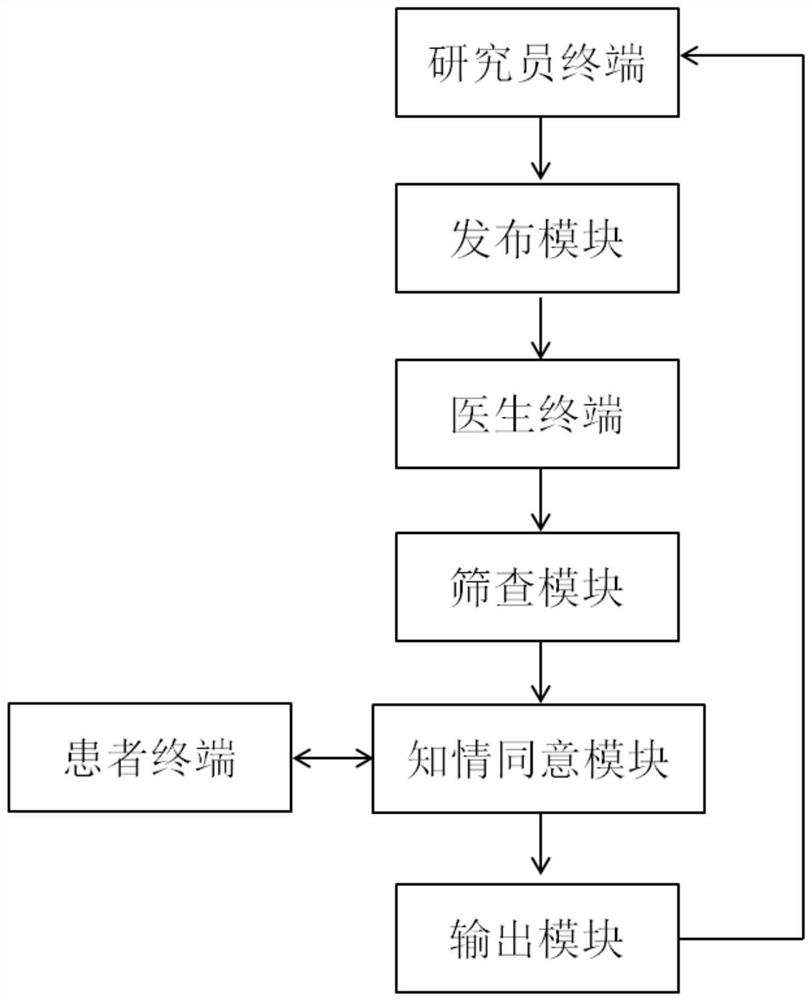
[0037] A clinical trial subject recruitment system such as figure 1 As shown, it includes researcher terminals, doctor terminals, patient terminals and servers, among which: researcher terminals, doctor terminals, and patient terminals are all mobile terminals; servers include:
[0038] The publishing module is used to obtain and publicize the project information initiated by the researcher's terminal; through publicizing the project information, the medical staff of all cooperative medical institutions can keep abreast of the disease situation and recruitment progress of the research project, which is convenient for them to dig out targetedly in the process of follow-up diagnosis and treatment suitable subjects;
[0039]The screening module is used to receive the recommended patient information sent by the doctor terminal according to the project information, and judge whether the patient meets the conditions according to the preset inclusion criteria and exclusion criteria, ...
Embodiment 2
[0049] Compared with Embodiment 1, the only difference is that the screening module is also used to compare patient information and inclusion criteria to obtain the degree of matching; and according to the preset recommended proportion information (set to 45% in this embodiment) Judging the degree of matching, if the degree of matching is greater than the recommended proportion, it is judged that the patient is eligible, the patient information is pushed to the researcher terminal, and the labor remuneration is calculated according to the degree of matching, and the labor remuneration is paid to the doctor. For example, if the matching degree of the recommended doctor reaches 50%, the patient information can be sent to the researcher; the follow-up researcher is responsible for follow-up, perfecting the selection, and finally being selected. Then you can get 50% of the recommended labor fee for recommending doctors; in this way, doctors can be motivated in a timely manner, mobi...
Embodiment 3
[0052] Compared with Embodiment 1, the only difference is that the patient terminal is also used to obtain the request file of the clinical trial procedure, interpret the request file, and generate detection instructions according to the keyword information in the standard file; In the clinical test of drugs, the requirement document stipulates that the subjects should not drink alcohol in their diet; therefore, after the requirement document is interpreted, the detection instruction generated based on the keyword "diet" is "during the meal time, the camera monitors the subject's The patient’s eating and drinking situation can be photographed”; the server is used to receive the detection instruction, and prompt the patient according to the detection instruction; the prompt means in this embodiment can be to send a push message to the patient terminal, and in other embodiments, similar The existing robotic phone technology is an existing technology, and will not be repeated here...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com