Preparation method and application of compound 3-hydroxy-3',4'-dihydroxy-phenethyl butyrate
A compound, phenethyl ester technology, applied in the fields of biology and medicine, can solve the problem of no brain fatigue research report, and achieve the effect of improving the ability of learning and memory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 3-Hydroxy-3', 4'-dihydroxy-butyric acid phenethyl ester (hydroxytyrosol hydroxybutyrate), its structure is shown in formula I:
[0061]
[0062] Preparation method one:
[0063]
[0064] Synthesis of S1, β-benzyloxybutyric acid (5)
[0065] Weigh crotonic acid (1) (2.55g, 29.7mmol) in a 150ml round bottom flask, add benzyl alcohol (3) (30ml), then add mercury acetate (9.63g, 30mmol), stir overnight at room temperature, put the flask into Cool the low-temperature condensation tank to 0°C, add 30ml of 3N sodium hydroxide within 5-10min, then add 30ml of 0.5M (0.57g) sodium borohydride in 3N sodium hydroxide aqueous solution, and keep the solution at 0°C for 3-10min. Take out and stir at room temperature for 1-2h, filter the filtrate, and extract with 75ml ether 3-4 times to remove excess benzyl alcohol. The aqueous layer was acidified to pH 2 with 10% hydrochloric acid, a large amount of white solids were precipitated, and β-benzyloxybutyric acid (5) was obtained ...
Embodiment 2
[0085] 1. Experimental materials
[0086] Hydroxytyrosol acetate was purchased from Santa Company of the United States, CAS No. 69039-02-7; ethyl β-hydroxybutyrate was purchased from Aladdin Company of the United States, CAS No. 24915-95-5.
[0087] 2. Experimental animal feeding and model establishment
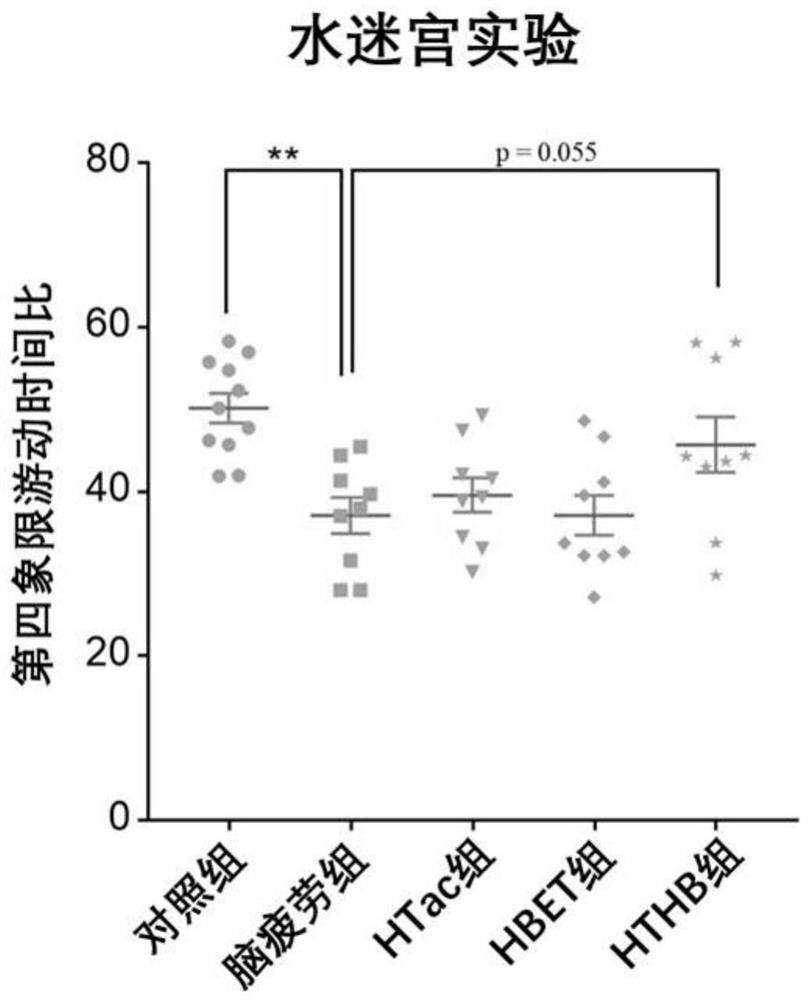
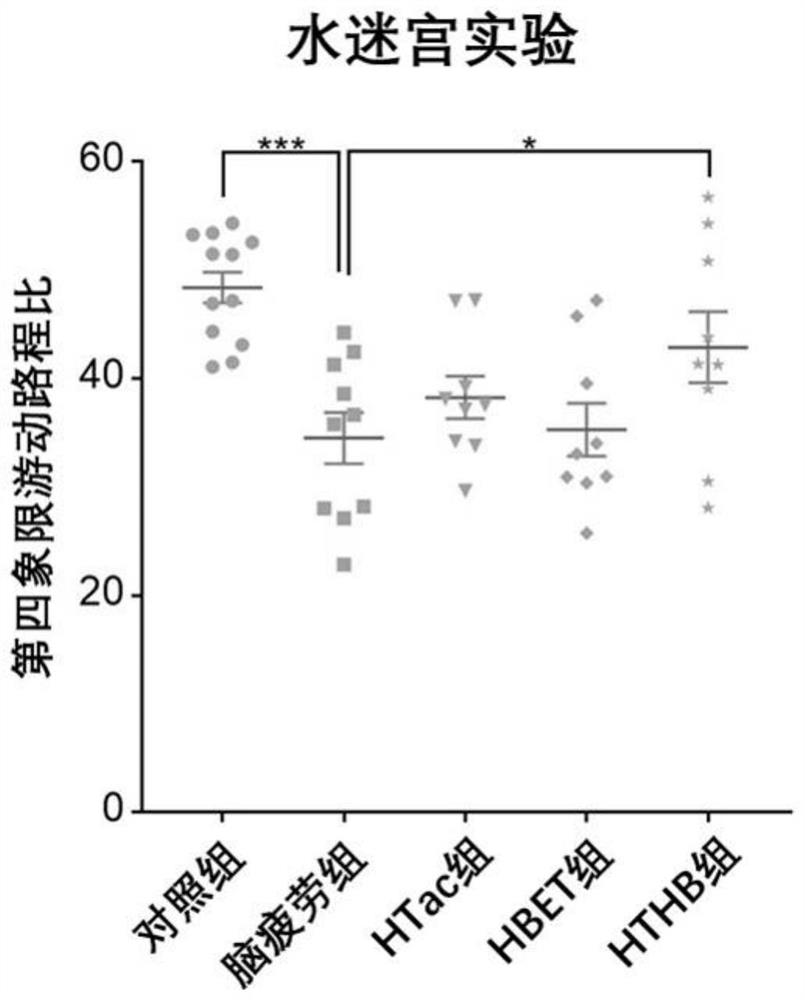
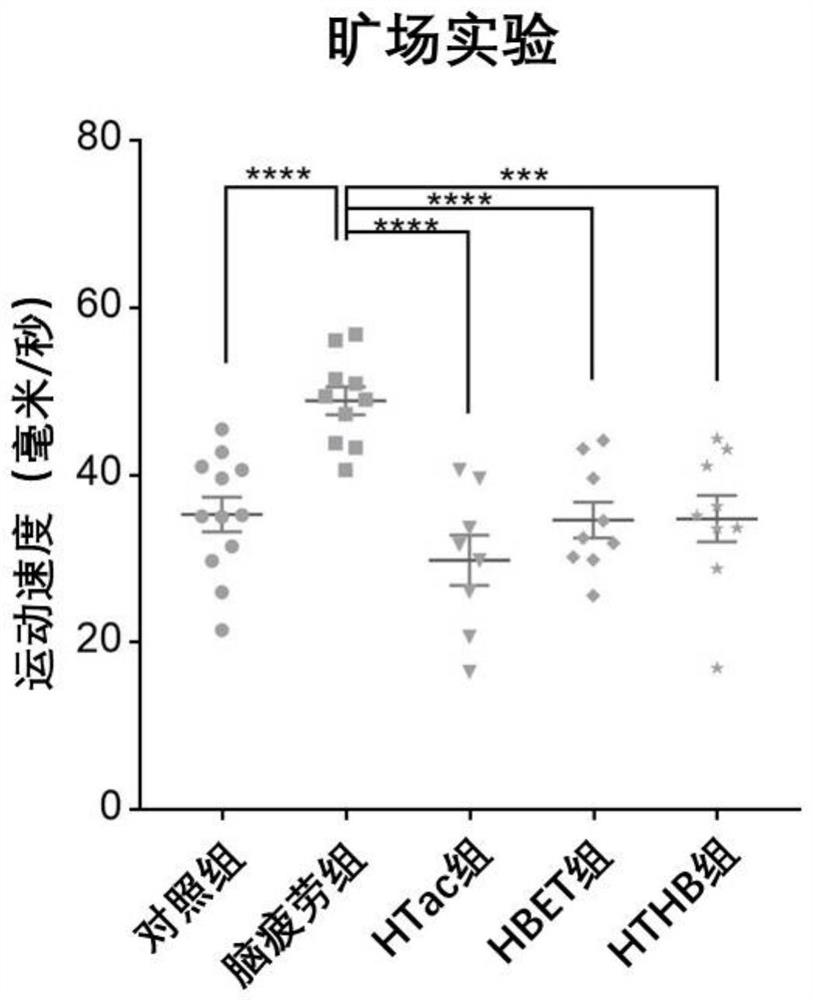
[0088] In this experiment, 8-week-old adult male SD rats of 250 g were purchased from the Animal Center of Shanghai Naval Military Medical University. The rats were kept in an animal room with controllable temperature (22°C to 28°C) and humidity (60%). The lights in the room were maintained in a 12-hour day and 12-hour night cycle, and the rats were free to eat during the experiment. and water ingress. In this experiment, sleep deprivation was used to establish a rat model of brain fatigue. The experimental rats were divided into five groups, 10 in each group. The five groups were: (1) the normal feeding group was fed normal saline every day at the same time (hereinafter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com