Preparation method of novel tinib medicine
A technology of tinib and drugs, applied in the field of medicine, can solve problems such as obvious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
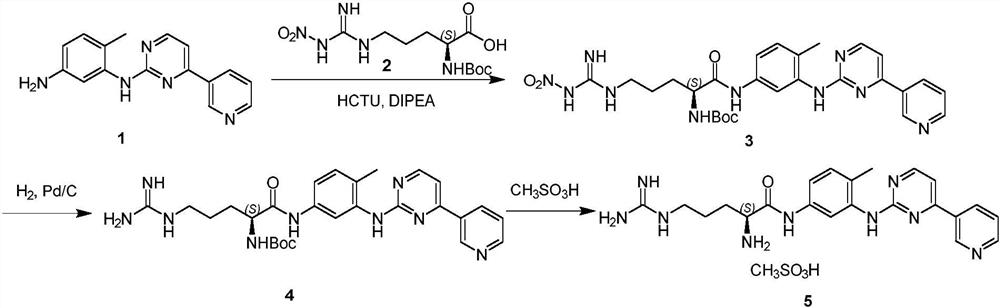
[0016] Add 27.7g (0.1mol, 1.0eq) N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2- Aminopyrimidine (1), 35.1g (0.11mol, 1.1eq) BOC-L-nitroarginine (2) and 250mL dimethylformamide (DMF), cooled to 10-20°C in an ice-water bath while stirring. Then add 45.5g (0.11mol, 1.1eq) of HCTU and 15.5g (0.12mol, 1.2eq) of DIPEA, and keep the reaction for 3 hours. The temperature was naturally raised to room temperature, and the reaction was continued for 2 h until the reaction of N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-aminopyrimidine (1) was completed. Add 500 mL of water to the reaction flask to quench the reaction, adjust the pH value to 9-10 with ammonia water, and precipitate a solid. After stirring for 1 hour, filter with suction, and rinse the obtained solid with 100 mL of water to obtain a crude product. Add the crude product into 200mL of ethyl acetate, heat to reflux to dissolve, cool down to 20-25°C with a water bath, stir and crystallize for 1 hour, filter with suction, and dry i...
Embodiment 2
[0020] Add 27.7g (0.1mol, 1.0eq) N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2- Aminopyrimidine (1), 38.3g (0.12mol, 1.2eq) BOC-L-nitroarginine (2) and 250mL dimethylformamide (DMF), cooled to 10-20°C in an ice-water bath while stirring. Then add 49.6g (0.12mol, 1.2eq) of HCTU and 18.1g (0.14mol, 1.4eq) of DIPE, and keep the reaction for 3 hours. The temperature was naturally raised to room temperature, and the reaction was continued for 2 h until the reaction of N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-aminopyrimidine (1) was completed. Add 500 mL of water to the reaction flask to quench the reaction, adjust the pH value to 9-10 with ammonia water, and precipitate a solid. After stirring for 1 hour, filter with suction, and rinse the obtained solid with 100 mL of water to obtain a crude product. Add the crude product into 200mL ethyl acetate, heat to reflux to dissolve, cool down to 20-25°C with a water bath, stir and crystallize for 1 hour, filter with suction, and dry in a ...
Embodiment 3
[0024] Add 27.7g (0.1mol, 1.0eq) N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2- Aminopyrimidine (1), 31.9g (0.1mol, 1.0eq) BOC-L-nitroarginine (2) and 250mL dimethylformamide (DMF), cooled to 10-20°C in an ice-water bath while stirring. Then add 45.5g (0.1mol, 1.1eq) HCTU and 15.5g (0.1mol, 1.2eq) DIPE, and keep the reaction for 3 hours. The temperature was naturally raised to room temperature, and the reaction was continued for 2 h until the reaction of N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-aminopyrimidine (1) was completed. Add 500 mL of water to the reaction flask to quench the reaction, adjust the pH value to 9-10 with ammonia water, and precipitate a solid. After stirring for 1 hour, filter with suction, and rinse the obtained solid with 100 mL of water to obtain a crude product. Add the crude product to 200mL ethyl acetate, heat to reflux to dissolve, cool down to 20-25°C with a water bath, stir and crystallize for 1 hour, filter with suction, and dry in a blast oven ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com