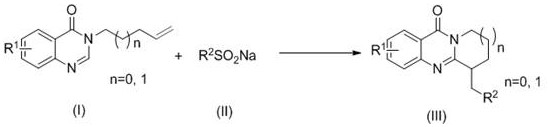
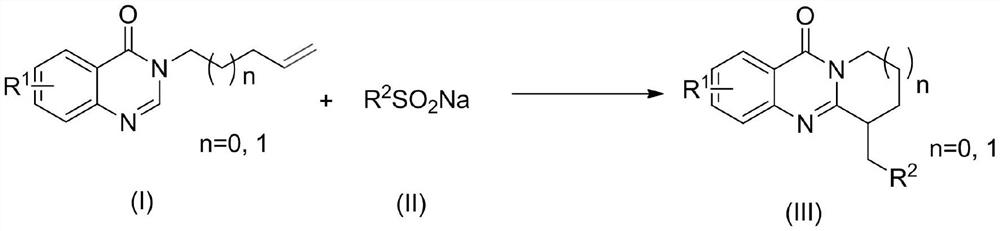
A kind of synthetic method of perfluoroalkyl substituted polycyclic quinazolinone derivatives
A polycyclic quinazolinone and perfluoroalkyl technology, which is applied in the field of synthesis of polycyclic quinazolinone derivatives, can solve the problems of harsh conditions, difficult preparation and the like, and achieves reaction safety, easy operation, and reaction selection. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 3-(pent-4-en-1-yl)quinazolinone (0.2mmol, 43mg), sodium trifluoromethanesulfinate (0.6mmol, 94mg) into a 10mL solvent storage bottle, add DMSO (2.0mL ) as a solvent, the reaction was carried out under open conditions, irradiated with 10w of visible light with a wavelength of 410-415nm and reacted at a temperature of 25°C for 8h. After the reaction, the reaction system was washed with water and extracted with dichloromethane, then separated into an organic layer and an aqueous layer. After the organic layer was dried with anhydrous sodium sulfate, it was evaporated and concentrated under reduced pressure to remove the solvent to obtain a yellow oil. The yellow oil was separated by column chromatography, using a mixture of petroleum ether and ethyl acetate with a volume ratio of 15:1 as the eluent, collecting the eluate containing the target compound, distilling off the solvent and drying to obtain 41 mg of white solid 6-( 2,2,2-trifluoroethyl)-8,9-dihydro-6hydro-pyri...
Embodiment 2
[0024] The experimental method of Example 2 was repeated in Example 1, except that "the solvent in the system was replaced with water (2ml)", and other operations were the same as in Example 1 to obtain 28 mg of white solid 6-(2,2,2- Trifluoroethyl)-8,9-dihydro-6hydro-pyrido[2,1-b]quinazolin-11(7hydrogen)-one, yield 50%.
Embodiment 3
[0026] The experimental method of embodiment 3 repeats embodiment 1, and difference is only " change sodium trifluoromethanesulfinate dosage into 0.4mmol ", other operations are the same as embodiment 1, obtain 38mg white solid 6-(2,2 ,2-trifluoroethyl)-8,9-dihydro-6hydro-pyrido[2,1-b]quinazolin-11(7hydrogen)-one, the yield was 67%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com