A lithium-sulfur secondary battery with high cycle stability and high coulombic efficiency
A lithium-sulfur secondary battery, cycle stability technology, applied in the direction of secondary batteries, secondary battery repair/maintenance, lithium batteries, etc., can solve the problems of destroying the solid phase reaction mechanism, positive electrode capacity decay, etc., to improve the utilization rate and lithium-sulfur battery capacity retention, the effect of inhibiting dissolution and alleviating the volume effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The lithium salt is selected from lithium bisfluorosulfonimide, the ether compound is selected from ethylene glycol dimethyl ether, and the compound containing the cyclic C=O structure is selected from ethylene carbonate. The concentration of lithium bisfluorosulfonimide was 1 mol / L, and ethylene carbonate accounted for 15 wt % of the total mass of ethylene glycol dimethyl ether and ethylene carbonate.
[0044] A lithium-sulfur battery is assembled using the above electrolyte, lithium metal negative electrode, sulfurized polyacrylonitrile positive electrode, and Celgard2400 separator. The specific operations are as follows:
[0045] Mix sulfided polyacrylonitrile, carbon nanotubes and polyvinylidene fluoride in N-methylpyrrolidone in a mass ratio of 8:1:1 to obtain a slurry with a concentration of 30%;
[0046] The slurry was evenly coated on the carbon-coated aluminum foil, dried at 60°C for 12 hours, and then cut into a 11mm diameter disc for a button battery or a 43m...
Embodiment 2~4
[0053] The assembly of the battery is similar to that of Example 1, except that the mass fraction of ethylene carbonate in the electrolyte is 5%, 10%, and 20%.
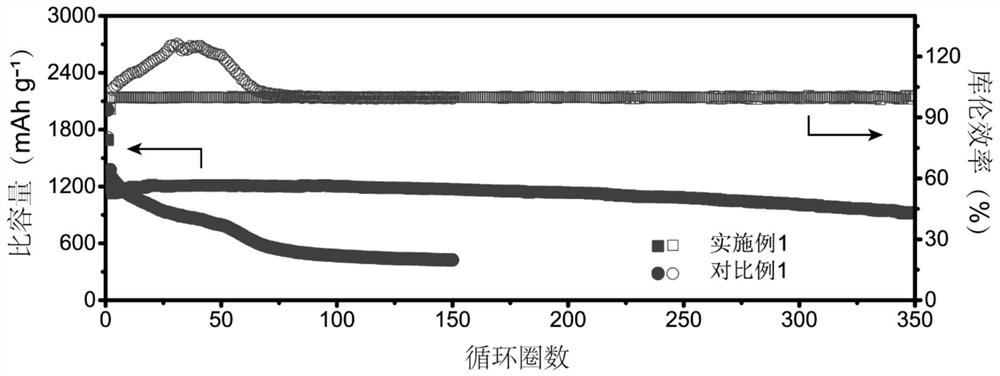
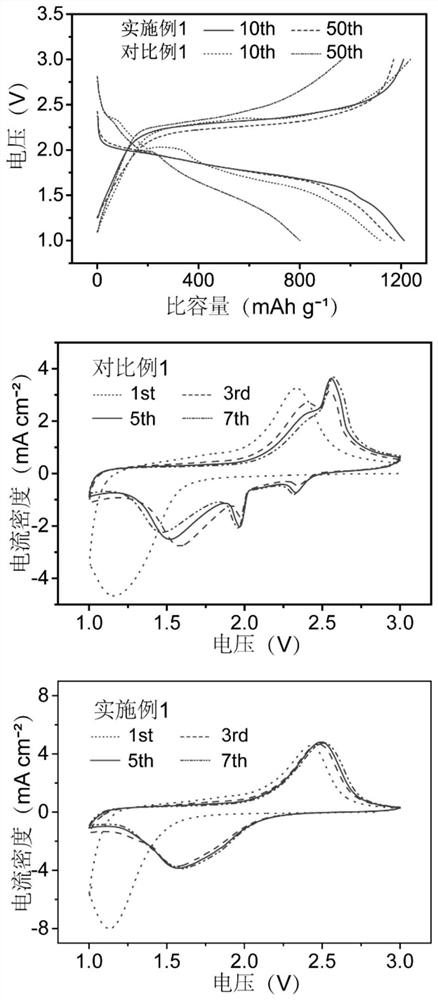
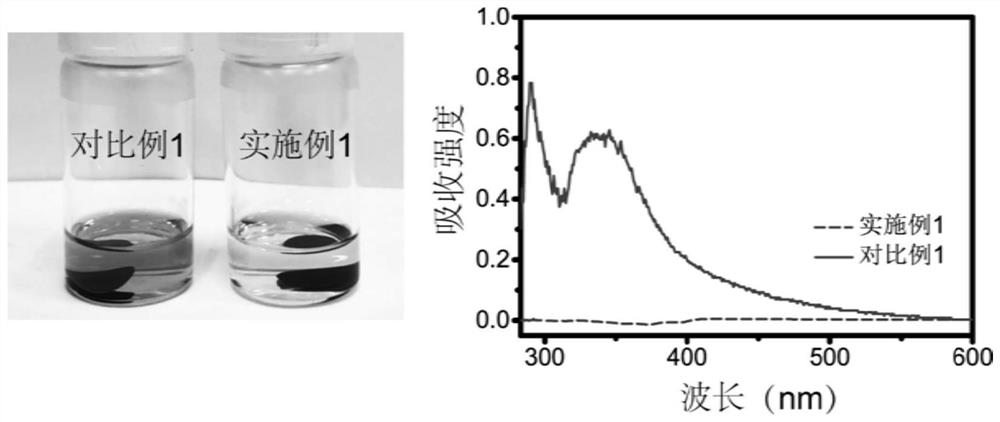
[0054] Under the condition that the charge-discharge rate is 0.5C, the batteries assembled with the electrolyte solutions provided in Examples 1-4 were tested for cycle performance. The charge-discharge voltage curve is as follows: Figure 4 As shown in the figure, the mass fraction of ethylene carbonate is 5% as EC05, the mass fraction of ethylene carbonate is 10% as EC10, the mass fraction of ethylene carbonate is 15% as EC15, and the mass of ethylene carbonate A score of 20% was recorded as EC20 (the same below). The charge-discharge voltage curves of the batteries assembled in each example showed a single-platform feature, which indicated the effectiveness of ethylene carbonate in transforming the reaction mechanism of vulcanized polyacrylonitrile. However, when the mass fraction of ethylene carbonate is 5%, the ...
Embodiment 5
[0058] The assembly of the battery is similar to that of Example 1, except that the lithium salt in the electrolyte is lithium bis-trifluoromethanesulfonimide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com