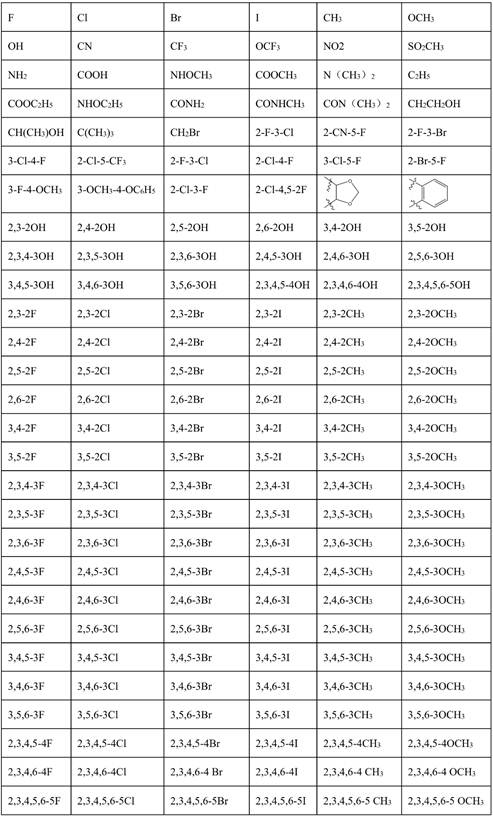
Macrolide brefeldin A ester derivative and application thereof
A kind of technology of feldsparin and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
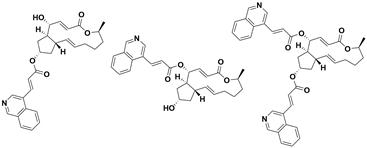
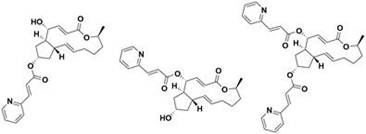
[0222] 7-O-(3-fluorocinnamic acid) acyl BFA (compound 1a) 4-O-(3-fluorocinnamic acid) acyl BFA (compound 1b)
[0223] 4,7-O-bis(3-fluorocinnamic acid)yl BFA (compound 1c)
[0224]
[0225] Under the protection of nitrogen, BFA (40mg, 1eq), DMAP (1eq) and EDC·HCl (4eq) were dissolved in 8ml of anhydrous DCM, stirred at 40°C for 10 min, and then the DCM solution of 3-fluorocinnamic acid (2eq) was added , continue to react for 2h, after the reaction is detected by TLC, water is added for extraction, the organic phase is concentrated under reduced pressure, the crude extract is separated and purified to obtain white solids 1a, 1b, and 1c, and the yields are 20%, 40%, and 30% in turn.
[0226] Structural Characterization: 1a: 1 H NMR (600 MHz, Chloroform- d ) δ = 7.59 (d, J =16.0, 1H),7.39 – 7.31 (m, 2H), 7.29 (s, 1H), 7.21 (dt, J =9.6, 2.0, 1H), 7.07 (td, J =8.3, 2.5, 1H), 6.39 (d, J =15.9, 1H), 5.93 (dd, J =15.6, 1.9, 1H), 5.73 (ddd, J =15.0, 10.2, 4.6, 1H), 5.32 – 5.1...
Embodiment 2
[0230] 7-O-(2-fluorocinnamic acid) acyl BFA (compound 2a) 4-O-(2-fluorocinnamic acid) acyl BFA (compound 2b)
[0231] 4,7-O-bis(2-fluorocinnamic acid)yl BFA (compound 2c)
[0232]
[0233] Referring to the preparation method of Example 1, white solids 2a, 2b, 2c were obtained. The yields were 20%, 45%, and 30%, respectively.
[0234] Structural Characterization: 2a: 1 H NMR (600 MHz, Chloroform- d ) δ = 7.78 (d, J =16.2, 1H),7.53 (td, J =7.6, 1.7, 1H), 7.39 – 7.32 (m, 2H), 7.16 (td, J =7.5, 1.1, 1H),7.10 (ddd, J =10.8, 8.2, 1.1, 1H), 6.50 (d, J =16.2, 1H), 5.93 (dd, J =15.7,2.0, 1H), 5.73 (ddd, J =15.0, 10.3, 4.6, 1H), 5.31 – 5.22 (m, 2H), 4.86 (dqd, J =12.6, 6.3, 1.7, 1H), 4.15 (dt, J =9.6, 2.5, 1H), 2.47 – 2.26 (m, 3H), 2.06 –1.81 (m, 5H), 1.78 – 1.64 (m, 2H), 1.58 – 1.48 (m, 1H), 1.26 (d, J =6.2, 3H),0.99 – 0.90 (m, 1H). 13 C NMR (150 MHz, Chloroform- d ) Δ = 166.6, 162.3, 160.6,151.6, 137.5, 136.1, 131.9, 131.1, 129.2, 124.6, 121.0, 117.9, 116.4,76.0, 71.9...
Embodiment 3
[0238] 7-O-(4-fluorocinnamic acid) acyl BFA (compound 3a) 4-O-(4-fluorocinnamic acid) acyl BFA (compound 3b)
[0239] 4,7-O-bis(4-fluorocinnamic acid)yl BFA (compound 3c)
[0240]
[0241] Referring to the preparation method of Example 1, white solids 3a, 3b, 3c were obtained. The yields were 20%, 33%, and 43%, respectively.
[0242] Structural characterization: Compound 3a: 1 H NMR (600 MHz, Chloroform- d ) δ = 7.61 (d, J =16.0,1H), 7.54 – 7.48 (m, 2H), 7.36 (dd, J =15.7, 3.1, 1H), 7.10 – 7.04 (m, 2H),6.32 (d, J =16.0, 1H), 5.93 (dd, J =15.6, 1.9, 1H), 5.73 (ddd, J =15.0, 10.2,4.6, 1H), 5.32 – 5.20 (m, 2H), 4.86 (dqd, J =12.5, 6.2, 1.8, 1H), 4.15 (dt, J =9.7, 2.3, 1H), 2.42 (qd, J =9.1, 7.0, 1H), 2.40 – 2.33 (m, 1H), 2.29 (dddd, J =12.9, 7.3, 3.3, 1.5, 1H), 2.02 (dddd, J =10.5, 8.3, 5.0, 2.7, 1H), 1.98 – 1.91(m, 1H), 1.91 – 1.82 (m, 2H), 1.78 – 1.71 (m, 1H), 1.72 – 1.62 (m, 2H), 1.53( dddd, J =14.5, 10.9, 7.3, 2.3, 1H), 1.26 (d, J =6.2, 3H), 0.99 – 0.89 (m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com