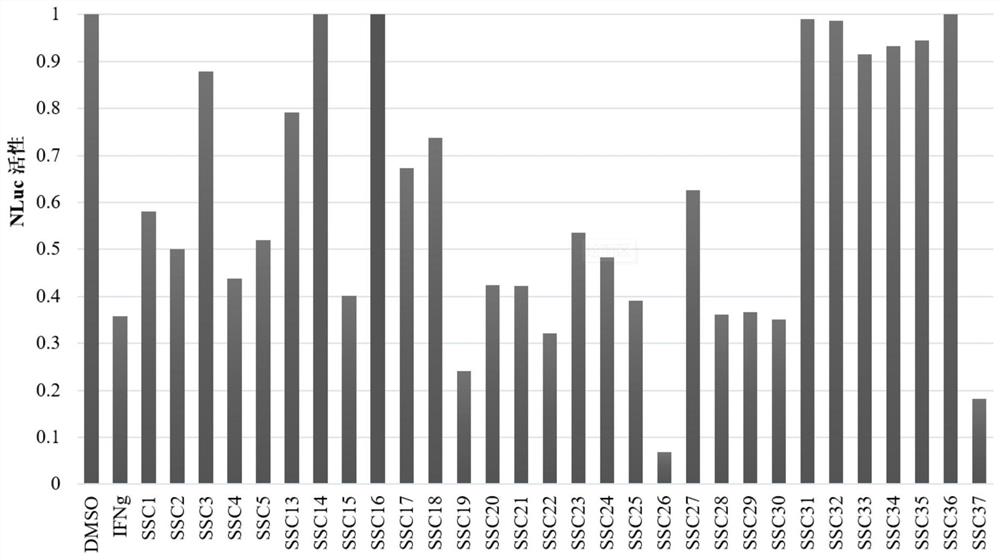
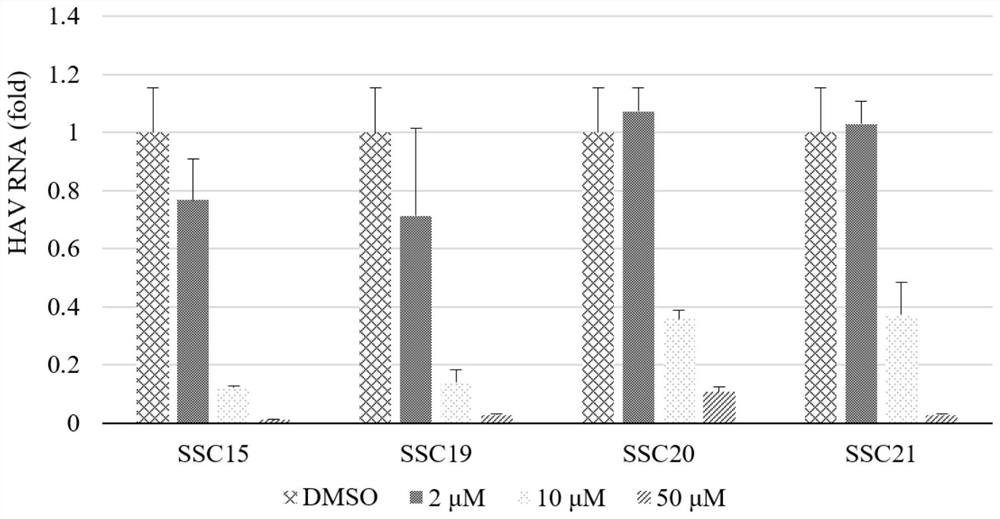
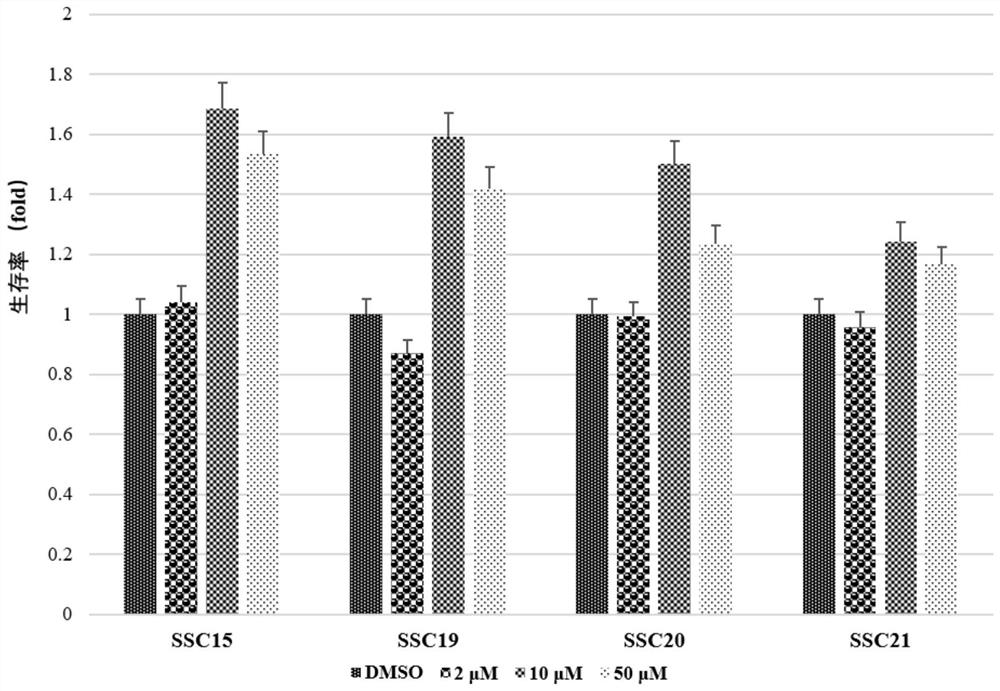
Polymethoxyflavone derivatives with anti-hepatitis A virus activity, preparation method and application thereof
A technology for polymethoxyflavonoids and hepatitis A virus, which is applied in the directions of antiviral agents, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problem of metabolic instability and limited content of polymethoxyflavonoids , low bioavailability and other problems, to achieve the effect of high research value and high clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of compound SSC2
[0058]
[0059] Add intermediate compound K (30mg, 0.074mmol), 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate into a 25mL eggplant-shaped bottle (28.1mg, 0.074mmol), diethylamine (9mg, 0.111mmol), N,N-diisopropylethylamine (9.5mg, 0.074mmol), dry DMF 10mL, react at 60°C for 10h. After the reaction was completed, it was extracted with water and saturated brine in sequence, and the organic layer was separated, dried, filtered and concentrated to obtain an oil, which was purified by silica gel column chromatography to obtain the target product SSC2.
[0060] The structural identification data are as follows: 1 H NMR (600MHz, CDCl 3 ):δ6.64(s,2H),6.22(d,J=2.35Hz,1H),6.09(d,J=2.35Hz,1H),4.86(dd,J=10.79,1.85Hz,1H),4.63 (s,2H)3.88(s,6H,OCH 3 ),3.85(s,3H,OCH 3 ),3.81(s,3H,OCH 3 ), 3.05(s,3H), 2.98(s,3H), 2.78(m,1H), 2.63(m,1H), 2.17(m,1H), 2.00(m,1H).
Embodiment 2
[0061] Embodiment 2: the preparation of compound SSC8
[0062]
[0063] Add intermediate compound K (30 mg, 0.074 mmol), tetramethoxysilane (22.5 mg, 0.148 mmol), p-chloroaniline (14 mg, 0.111 mmol), toluene 10 mL, and react at 100 ° C for 10 h in a 25 mL eggplant-shaped bottle. After the reaction was completed, it was extracted with water and saturated saline in sequence, the organic layer was separated, dried, filtered and concentrated to give a light yellow powder, which was purified by silica gel column chromatography to obtain the target product SSC8.
[0064] The structural identification data are as follows: 1 H NMR (600MHz, CDCl 3 ):δ8.22(s,N-H),7.55(d,J=7.94Hz,2H),7.05(d,J=22.61Hz,2H),6.64(s,2H),6.19(d,J=2.37Hz ,1H),6.17(d,J=2.37Hz,1H),4.90(dd,J=10.70,1.90Hz,1H),4.57(s,2H),3.88(s,6H,OCH 3 ),3.85(s,3H,OCH 3 ),3.84(s,3H,OCH 3 ),2.80(ddd,J=16.80,5.57,2.37Hz,1H),2.65(m,1H),2.20(ddt,J=13.59,6.11,2.18Hz,1H),2.01(dtd,J=13.71,11.53 ,5.67Hz,1H).
Embodiment 3
[0065] Embodiment 3: the preparation of compound SSC15
[0066]
[0067] Add intermediate compound L (30 mg, 0.072 mmol), 5-chlorothiophene-2-carbaldehyde (16.0 mg, 0.111 mmol), 10 mL of ethanol, and catalytic amount of glacial acetic acid into a 25 mL eggplant-shaped bottle, and heat to reflux for 10 h. After the reaction was completed, it was extracted with water and saturated brine in sequence, the organic layer was separated, dried, filtered and concentrated to obtain an oily compound, which was purified by silica gel column chromatography to obtain the target product SSC15.
[0068] The structural identification data are as follows: 1 H NMR (600MHz, DMSO-d 6 ): δ11.62 (ds, 1H, isomer a), 11.56 (ds, 1H, isomer b), 8.48 (s, 1H, isomer a), 8.07 (s, 1H, isomer b ), 7.34 (d, 1H, J = 3.98Hz isomer a), 7.32 (d, 1H, J = 3.95Hz isomer b), 7.16 (d, 1H, J = 3.94Hz isomer a), 7.13 (d, 1H, J = 3.94Hz isomer b), 6.72 (s, 4H, isomer a, b), 6.25 (d, J = 2.28Hz, 1H, isomer a), 6.17 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com