Photoprotective compositions containing malassezia-derived compounds and/or chemical analogs thereof
A technology of compounds and analogs, applied in the field of photoprotective compositions containing compounds derived from Malassezia (MALASSEZIA) and/or their chemical analogs, capable of solving problems such as harmful ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0256] Compound name
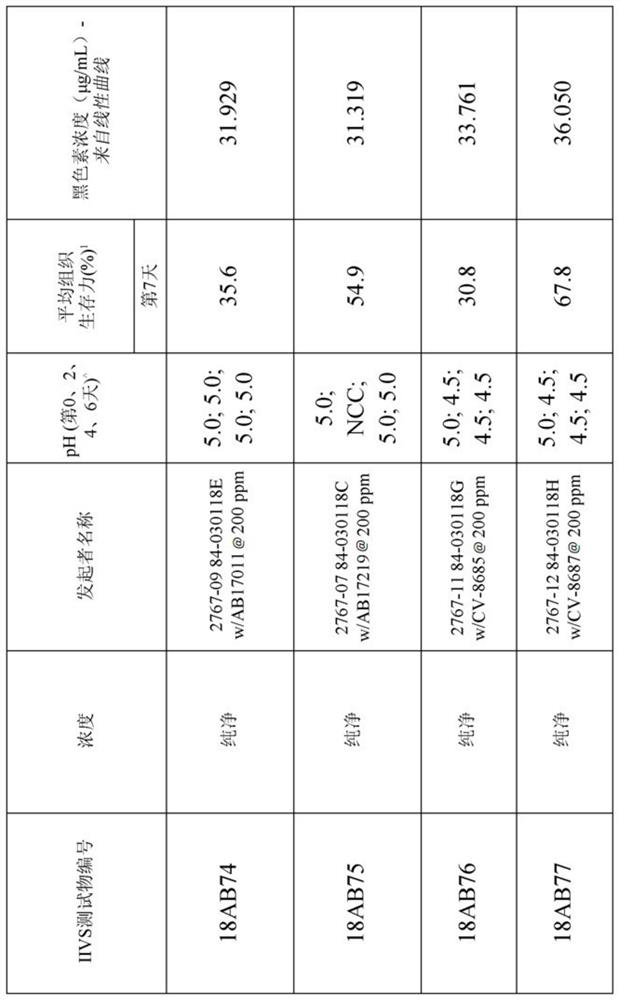
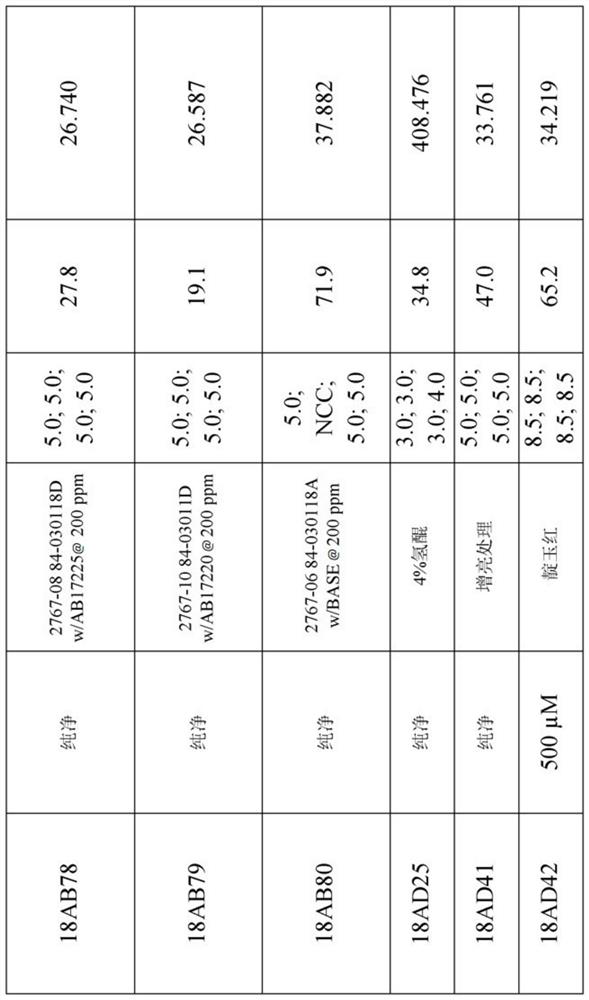
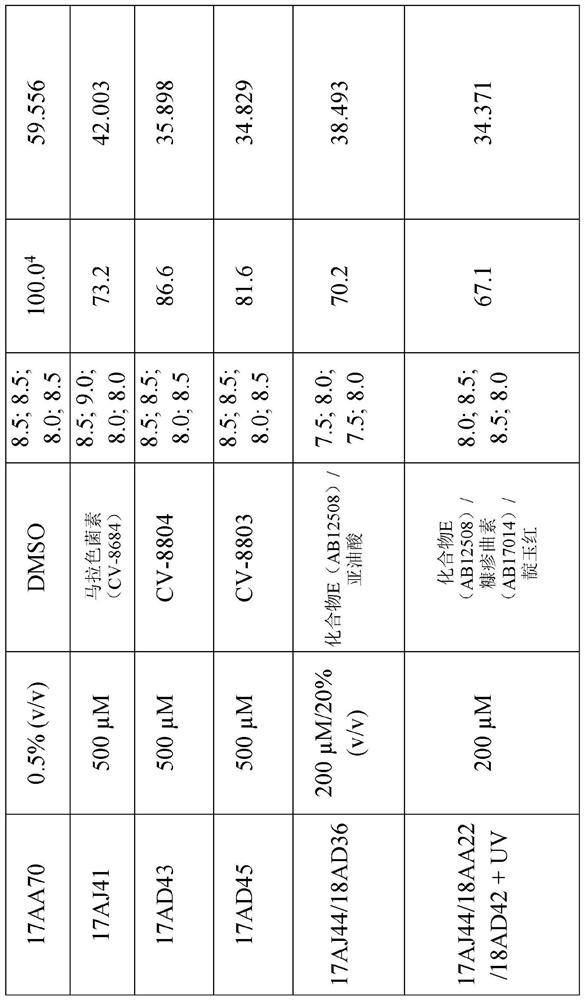
[0257] Table 1 below shows the structures and names of the compounds of the present invention.
[0258] Table 1
[0259]
[0260]
[0261]
[0262]
[0263]
[0264]
[0265]
[0266]
Embodiment 2
[0268] Apoptosis Inducing Activity of Indirubin and Indirubin Derivatives
[0269] Reagent
[0270] Alexa Fluor 488 Annexin V / Dead Cell Apoptosis Kit, Fetal Bovine Serum (FBS), 0.25% Trypsin-EDTA (1×), Caspase-Glo 3 / 7 Assay, RPMI 1640 Medium , Dulbecco's Modified Eagle Medium and antibiotic antimycotic solution (100×).
[0271] The cell line MeWo( HTB-65 TM ), WM115 ( CRL-1675) and B16F1 ( CRL-6323) were maintained in the following media: media for MeWo and B16F1: DMEM supplemented with 10% FBS; media for WM115: RPMI 1640 supplemented with 10% FBS.
[0272] experimental method
[0273] Cells were harvested and cell numbers determined using a Countess cell counter. Dilute the cells to the desired density with culture medium. For example, the final cell density can be 4,000 cells / well for 6 hour and 24 hour treatments, and 2,000 cells / well for 48 hour and 72 hour treatments. For Annexin V assays, 384-well clear-bottom plates (Corning 3712) were used, while for Casp...
Embodiment 3
[0280] Cell Viability After Exposure to Indirubin and Indirubin Derivatives
[0281] Reagent
[0282] CellTiter- 2.0 Analysis.
[0283] experimental method
[0284] For CellTiter-Glo assays, test compounds were prepared in 10 mM DMSO solutions. Compounds were serially diluted into 12 concentrations. 40 μL of cells from the 100,000 cells / ml suspension was dispensed into each well of a 384-well plate (Corning3570). Incubate the plate at 37 °C, 5% CO 2 and 95% humidity overnight. Test compounds were added, with DMSO as vehicle control. Incubate the plate at 37 °C, 5% CO 2 and 95% humidity for 6, 24 or 48 hours, and 40 μL of CellTiter-Glo reagent was added to the wells to analyze cell viability.
[0285] result
[0286] Compounds and compositions of the invention, including indirubin and its chemical analogs, are expected to induce cell death. Chemical analogues of indirubin are expected to exhibit, for example, stronger apoptosis-inducing activity than indirubin. Lik...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com