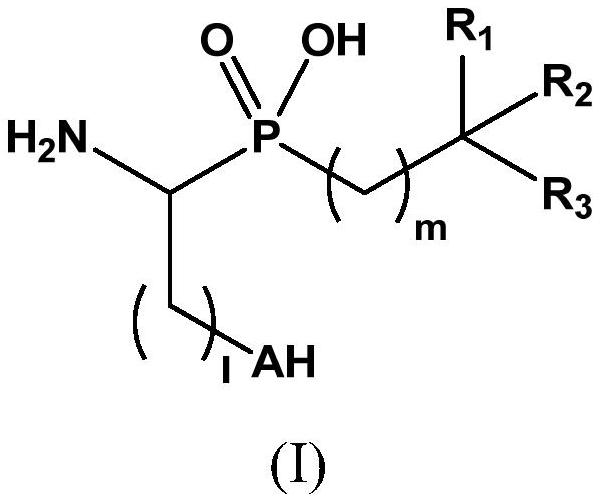
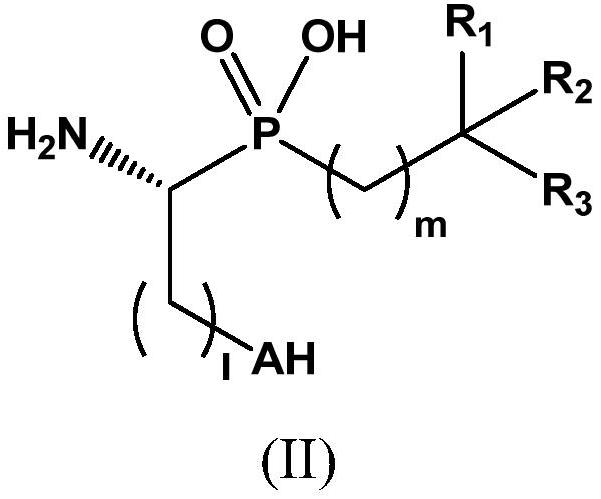
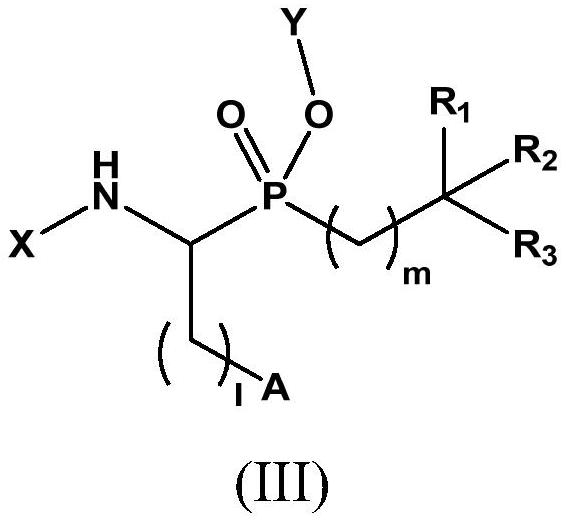
Novel aminophosphinic derivatives as aminopeptidase a inhibitors
A technology of amino and group, which is applied in the field of preparation of the compound, can solve problems such as increased activity and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220] Example 1: 4-Amino-4-[hydroxy(3-methylbutyl)phosphoryl]butanoic acid
[0221] Step 1: (3-Methylbutyl)phosphinic acid
[0222] According to protocol A from anhydrous Et 2 Diethyl chlorophosphite (1.90 mL, 17.4 mmol, 1.0 eq.) in O (6 mL), followed by addition from anhydrous Et 2 The title compound (1.40 g, 59%) was prepared from freshly prepared Grignard reagent in 1-bromo-3-methylbutane (2.76 g, 18.3 mmol, 1.05 eq.) in O (9 mL).
[0223] MS (ESI + ): [M+H] + =137.2; [(Mx2)+H] + =273.2
[0224] 1 H NMR (MeOD, 500MHz) δ (ppm): 7.02 (dt, J=536.2, 2.0Hz, 1H); 1.85-1.71 (m, 2H); 1.71-1.59 (m, 1H); 1.55-1.42 (m, 2H); 0.96(d, J=6.7Hz, 6H)
[0225] 31 P NMR (CD 3 OD, 202MHz) δ (ppm): 36.32
[0226] Step 2: [4-(Benzyloxy)-1-{[(benzyloxy)carbonyl]amino}-4-oxobutyl](3-methylbutyl)phosphinic acid
[0227] From the previous product (800 mg, 5.88 mmol, 1.0 eq.) and NH in AcOH (10 mL) and AcCl (1.2 mL) according to Protocol B of multicomponent reactions 2 Cbz (977mg, 6.46m...
Embodiment 2
[0237] Example 2: 4-Amino-4-[hydroxy(4-methylpentyl)phosphoryl]butanoic acid
[0238] Step 1: (4-Methylpentyl)phosphinic acid
[0239] According to protocol A from anhydrous Et 2 Diethyl chlorophosphite (1.26 mL, 11.5 mmol, 1.0 eq.) in O (6 mL), followed by addition of 2 1-Bromo-4-methylpentane (2.0 g, 12.1 mmol, 1.05 eq.) in O (6 mL) freshly prepared Grignard reagent to prepare the title compound (740 mg, 43%).
[0240] MS (ESI + ): [M+H] + =151.2; [(Mx2)+H] + =301.2
[0241] 1 H NMR (500MHz, MeOD) δ (ppm): 7.01 (dt, J = 536.1, 2Hz, 1H); 1.78-1.67 (m, 2H); 1.67-1.53 (m, 3H); 1.35-1.27 (m, 2H ); 0.91 (d, J=6.6Hz, 6H)
[0242] 31 P NMR (CD 3 OD, 202MHz) δ (ppm): 35.69
[0243] Step 2: [4-(Benzyloxy)-1-{[(benzyloxy)carbonyl]amino}-4-oxobutyl](4-methylpentyl)phosphinic acid
[0244] From the previous product (300 mg, 2.0 mmol, 1.0 eq.) and NH in AcOH (5 mL) and AcCl (428 μL) according to Protocol B of the multicomponent reaction 2 Cbz (362 mg, 2.4 mmol, 1.2 eq.), f...
Embodiment 3
[0255] Example 3: 4-Amino-4-[hydroxy(5-methylhexyl)phosphoryl]butanoic acid
[0256] Step 1: (5-Methylhexyl)phosphinic acid
[0257] According to protocol A from anhydrous Et 2 Diethyl chlorophosphite (1.15mL, 10.54mmol, 1.0eq.) in O (6mL) was subsequently added from anhydrous Et 2 1-Bromo-5-methylhexane (2.0 g, 11.17 mmol, 1.05 eq.) in O (5 mL) freshly prepared Grignard reagent to prepare the title compound (797 mg, 46%).
[0258] MS (ESI + ): [M+H] + =165.2; [(Mx2)+H] + =329.2
[0259] 1 H NMR (500MHz, MeOD) δ (ppm): 7.00 (dt, J = 533.5, 1.99Hz, 1H); 1.73 (s, 2H); 1.62-1.51 (m, 3H); 1.43 (dd, J = 8.6, 7.5Hz, 2H); 1.23(dd, J=8.6, 7.0Hz, 2H); 0.90(d, J=6.6Hz, 6H)
[0260] 31 P NMR (CD 3 OD, 202MHz) δ (ppm): 35.5
[0261] Step 2: [4-(Benzyloxy)-1-{[(benzyloxy)carbonyl]amino}-4-oxobutyl](5-methylhexyl)phosphinic acid
[0262] From the previous product (300 mg, 1.83 mmol, 1.0 eq.) and NH in AcOH (4 mL) and AcCl (391 μL) according to Protocol B of the multicomponent re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com